Abstract
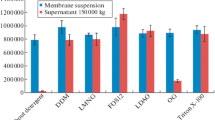
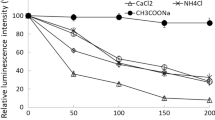
The authentic recombinant luciferase, the luciferase with the structure similar to that of the native protein, was obtained using random mutagenesis, and its properties were studied in comparison with several fusion proteins. Thermoinactivation curves of the recombinant luciferases within the 10–50°C temperature interval showed that thermoinactivation involves reversible and irreversible steps. Immobilization of the recombinant Luciola mingrelica and Photinus pyralis firefly luciferases on BrCN-activated sepharose was carried out. Immobilization resulted in the preparation of enzymes with high catalytic activity. Physicochemical properties and analytical characteristics of the immobilized recombinant and native luciferases were studied. The catalytic properties of the immobilized recombinant L. mingrelica luciferase were close to those of the native luciferase but the former enzyme appeared to be significantly more stable. The immobilized recombinant luciferases can be used for ATP assay within 0.01–10000 nM range.
Similar content being viewed by others
References
Ugarova, N. N., Brovko, L. Yu., and Kutuzova, G. D. (1993), Biochemistry (Moscow) 58, 976–992.
Devine, J. H., Kutuzova, G. D., Green, V. A., Ugarova, N. N., and Baldwin, T. O. (1994), Biochim. Biophys. Acta 1173, 121–132.
Dementieva, E. I., Kutuzova, G. D., Zheleznova, E. E., Lundovskikh I. A., and Ugarova, N. N. (1996), Biochemistry (Moscow) 61, 115–119.
Kutuzova, G. D., Skripkin, E. A., Belogurova, N. G., Skrjabin G. A., Ugarova, N. N., and Varfolomeev, S. D. (1990), Dokl. Akad. Nauk SSSR 314, 757–761.
Dementieva, E. I., Kutuzova, G. D., and Ugarova, N. N. (1989), Moscow Universitet Chem. Bull. (Engl. trans.) 44, 69–73.
Talebarovskava, I. K., Katkova, V. A., Rizhova, V. V., Schogolev, A. A., and Berezin, I. V. (1983), Method of D-luciferin synthesis. USSR patent no. 1192324.
Lee, J., Jablonski, I., and DeLuca M. (1977), Anal. Biochem. 80, 496–501.
Blum, L. J., Coulet, P. R., and Gauteron, D. C. (1985), Biotech. Bioeng. 27, 232–237.
Roda, A., Grigolo, B., Girotti, S., Ghini, S., Carrea, G., and Bovara, R. (1991), in Bioluminescence and Chemiluminescence. (Stanley, P. E. and Kricka, L. J., eds.), Wiley, Chichester, pp. 487–489.
Ugarova, N. N., Brovko, L. Yu., and Kost N. N. (1982) Enz. Microb. Technol. 4, 224–228.
Wang, C. and Andrade, J. (1994), in Bioluminescence and Chemiluminescence. (Campbell, A. K., Kricka, L. J., and Stanley, P. E., eds.), Wiley, Chichester, pp. 423–426.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lundovskikh, I., Dementieva, E. & Ugarova, N. Recombinant firefly luciferase in Escherichia coli . Appl Biochem Biotechnol 88, 127–136 (2000). https://doi.org/10.1385/ABAB:88:1-3:127
Issue Date:
DOI: https://doi.org/10.1385/ABAB:88:1-3:127