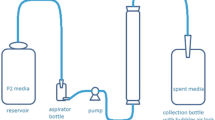
Abstract
Clostridium beijerinckii BA101 (mutant strain) and C. beijerinckii 8052 (wild type) were compared for substrate and butanol inhibition. The wild-type strain is more strongly inhibited by added butanol than is the mutant strain. Acetone and butanol were removed from a fed-batch reactor inoculated with C. beijcrinckii BA101 by pervaporation using a silicone membrane. In the batch reactor, C. beijerinckii BA101 produced 25.3 g/L of total solvents, whereas in the fermentation-recovery experiment it produced 165.1 g/L of total solvents. Solvent productivity increased from 0.35 (batch reactor) to 0.98 g/L·h (fed-batch reactor). The fed-batch reactor wasfed with 500 g/L of glucose-based P2 medium. Acetone selectivities ranged from 2 to 10 whereas butanol selectivities ranged from 7 to 19. Total flux varied from 26 to 31 g/m2·h.
Similar content being viewed by others
Abbreviations
- A :
-
Pervaporation membrane area (m2)
- C g :
-
Glucose concentration in concentrated feed medium (C g1 and C g2 for first and second additions, respectively) (g/L)
- C g0 :
-
Glucose concentration in fermentation reactor at 0 time (g/L)
- C ge :
-
Glucose concentration in fermentation broth at the end of fermentation (g/L)
- G gn :
-
Glucose concentration in nth addition of concentrated feed medium (n=1,2,3,… n) (g/L)
- C s :
-
Solvent concentration in condensate (C s1 and C s2 for first and second condensates, respectively) (g/L)
- C af :
-
Solvent concentration in fermentation broth at the end of pervaporation (g/L)
- C sn :
-
Solvent concentration in nth condensate (n=1,2,3, … n) (g/L)
- H :
-
Time period during which pervaporation membrane condensate was collected (h)
- R p :
-
Reactor/solvent productivity (g/L·h)
- T :
-
Fermentation time (h)
- V c :
-
Volume of pervaporation membrane condensate (V c1 and V c2 for first and second condensates, respectively) (L)
- V cv :
-
Volume of nth condensate of pervaporation membrane (n=1,2,3,… n) (L)
- V fD :
-
Volume of fermentation broth at 0 time (L)
- V fc :
-
Volume of fermentation broth at the end of fermentation (L)
- V fc :
-
Volume of fermentation broth at the end of pervaporation (L)
- V g :
-
Volume of concentrated feed medium added to the fed-batch reactor (L)
- V gn :
-
Volume of nth addition of concentrated feed medium to the fedbatch reactor (n=1,2,3,…n) (L)
- W :
-
Weight of condensate collected from the pervaporation membrane (g)
- X :
-
Weight fraction of butanol or total solventin fermentation broth(−)
- Y :
-
Weight fraction of butanol or total solvent in the membrane permeate (condensate) (−)
- Y p/B :
-
Yield of solvents (g solvents/g glucose utilized)
- α:
-
Selectivity of acetone/butanol or total solvents (−)
References
Woods, D. T. and Woods, D. R. (1986), Microbiol. Rev. 50, 484–524.
McNeil, B. and Kristiansen, B. (1986), Adv. Appl. Microbiol. 31, 61–92.
Maddox, I. S. (1989), Biotechnol. Genet. Eng. Rev. 7, 189–220.
Parekh, M., Formanek, J., and Blaschek, H. P. (1998), J. Ind. Microbiol. Biotechnol. 21, 187–191.
Annous, B. A. and Blaschek, H. P. (1991), Appl. Environ. Microbiol. 57, 2544–2548.
Formanek, J., Mackie, J. R., and Blaschek, H. P. (1997), Appl. Environ. Microbiol. 63, 2306–2310.
Chen, C.-K. and Blaschek, H. P. (1999), Appl. Microbiol. Biotechnol. 52, 170–173.
Wagner, J. J., Lusby, K. S., and Horn, G. W. (1983), J. Anim. Sci. 57, 542–552.
Parekh, M., Formanek, J., and Blaschek, H. P. (1999), Appl. Microbiol. Biotechnol. 51, 152–157.
Qureshi, N., Maddox, I. S., and Friedl, A. (1992), Biotechnol. Prog. 8, 382–390.
Qureshi, N. (1997), PhD thesis, University of Nebraska, Lincoln, NE.
Qureshi, N. and Blaschek, H. P. (1999), Separation Sci. Technol. 34, 2803–2815.
Qureshi, N. and Blaschek, H. P. (1999), Biotechnol. Prog. 15, 594–602.
Qureshi, N. and Blaschek, H. P. (1999), Biomass Bioenergy 17, 175–184.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qureshi, N., Blaschek, H.P. Butanol production using Clostridium beijerinckii BA101 hyper-butanol producing mutant strain and recovery by pervaporation. Appl Biochem Biotechnol 84, 225–235 (2000). https://doi.org/10.1385/ABAB:84-86:1-9:225
Issue Date:
DOI: https://doi.org/10.1385/ABAB:84-86:1-9:225