Abstract
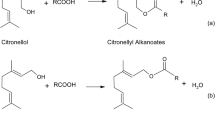
The production of low molecular weight esters as flavor compounds by biotechnological processes has a potential interest for the food industry. The use of natural available substrates and enzymes is an essential part of the process design, because the products may obtain natural label. In this study, direct esterification of citronellol and geraniol with short-chain fatty acids catalyzed by free lipase from Mucor miehei was performed with high yields in n-hexane. The effects of the acid:alcohol ratio on the bioconversion rate of increasing chain length esters was investigated. To reach the optimum yield, substrates and enzyme concentration were determined. The inhibiting effects of acid are strongly attenuated by reducing the quantity of acid and increasing the amount of enzyme in media following the optimum values. Improvements have been made to increase the ester purity. The consumption of excess substrate by adding calculated amounts of acid gives a 10% yield enhancement, and leads to 100% pure terpenyl esters. The first steps to a scale-up application were attempted using a reactor that allowed us to produce ester quantities up to 100 cm3. Separation and purification of the products were treated with success, underlining the lipase stability and efficiency under the conditions of this study. The ability to recover the enzyme, and reusing it in bioconversions, plays a major role in reducing the cost of the overall process.
Similar content being viewed by others
References
Langrand, G., Triantaphylides, C., and Baratti, J. (1988), Biotechnol. Lett. 10, 549–554.
Langrand, G., Rondot, N., Triantaphylides, C., and Baratti, J. (1990), Biotechnol. Lett. 12, 581–586.
Welsh, F. W., Williams, R. E., and Dawson, K. (1990), J. Food Science 55, 1679–1682.
Brady, L., Brzozanski, A. M., Derewenda, Z. S., Dodson, E., Dodson, G., Tolley, S., Turkenburg, J. P., Christiansen, L., Huge-Jensen, B., Norskov, L., Thim, L., and Menge, U. (1990), Nature 343, 767–770.
Brenner, R. H., Halling, P. J., and Bell, G. (1988), Biotechnol. Lett. 334, 528–530.
Valivety, R. H., Halling, P. J., and Macrae, A. R. (1992a), Biochim. Biophys. Acta 1118, 218–222.
Valivety, R. H., Halling, P. J., and Bell, G. (1991), Biotechnol. Lett. 15, 1133–1138.
Iwai, M., Okumura, S., and Tsujisaka, Y. (1980), Agric. Biol. Chem. 44, 2731, 2732.
de Ćastro, H. F., Anderson, W. A., Moo-Young, M., and Legge, R. L. (1992), in Biocatalysis in Non-Conventional Media, Tramper, J., Vermùe, M. H., and Beeftink, H. H., eds., Elsevier, New York, p. 475.
Claon, P. A. and Akoh, C. C. (1993), Biotechnol. Lett. 12, 1211–1216.
Fonteyn, F., Blecker, C., Lognay, G., Marlier, M., and Severin, M. (1994), Biotechnol. Lett. 16, 693–696.
Yee, L. N., Akoh, C. C., and Phillips, R. S. (1995), Biotechnol. Lett. 17, 67–70.
Stamatis, H., Christakopoulos, P., Kekos, D., Macris, B. J., and Kolisis, F. N. (1998), J. Mol. Catal. B: Enzyme 4, 229–236.
Nakagawa, H., Watanabe, S., Shimura, S., Kirimura, K., and Usami, S. (1998), World J. Microbiol. Biotechnol. 14, 219–222.
Chatterjee, T. and Bhattacharyya, D. K. (1998), Biotechnol. Lett. 20, 865–868.
Molinari, F., Villa, R., and Aragozzini, F. (1998), Biotechnol. Lett. 20, 41–44.
Oguntimein, G. B., Anderson, W. A., and Moo-Young, M. (1995), Biotechnol. Lett. 17, 77–82.
De Castro, H. F., Napoleao, D. A. S., and De Oliveira, P. C. (1998), Appl. Biochem. Biotechnol. 70–72, 667–675.
Perraud, R., Moreau, L., and Krahé, E. (1995), Deutsche Lebensmittel-Rundschau 91, 219–221.
Perraud, R. and Laboret, F. (1995), Appl. Microbiol. Biotechnol. 44, 321–326.
Claon, P. A. and Akoh, C. C. (1993), Biotechnol. Lett. 15, 1211–1216.
Claon, P. A. and Akoh, C. C. (1994), Enzyme Microb. Technol. 16, 835–838.
Akoh, C. C. and Yee, L. N. (1998), J. Mol. Catal. B: Enzyme 4, 149–153.
Takahashi, K., Saito, Y., and Inada, Y. (1988), J. Am. Oil Chem. Soc. 65, 911–916.
Chulalaksananukul, W., Condoret, S., and Combes, D. (1992), Enzyme Microb. Technol. 14, 293–298.
Chulalaksananukul, W., Condoret, S., and Combes, D. (1993), Enzyme Microb. Technol. 15, 691–698.
Manjòn, A., Iborra, J. L., and Arocas, A. (1991), Biotechnol. Lett. 13, 339–344.
Borzeix, F., Monot, F., and Vandecasteele, J. P. (1992), Enzyme Microb. Technol. 4, 791–797.
Okumura, S., Iwai, M., and Tsujisaka, Y. (1979), Biochim. Biophys. Acta 575, 156–165.
Laane, C., Boeren, S., and Vos, K. (1985), Trends Biotechnol. 3, 251, 252.
Laane, C., Boeren, S., Vos, K., and Veeger, C. (1987), in Biocatalysis in Organic Media, Laane, C., Tramper, J., and Lilly, M. D., eds., Elsevier, New York, p. 65.
Zaks, A. and Klibanov, A. M. (1988), J. Biol. Chem. 263, 8017–8021.
Dordick, J. S. (1989), Enzyme Microb. Technol. 11, 194–211.
Claon, P. A. and Akoh, C. C. (1994), J. Am. Chem. Soc. 71, 575–578.
Laboret, F. (1996), PhD thesis, Université Joseph Fourier, Grenoble 1, France.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laboret, F., Perraud, R. Lipase-catalyzed production of short-chain acids terpenyl esters of interest to the food industry. Appl Biochem Biotechnol 82, 185–198 (1999). https://doi.org/10.1385/ABAB:82:3:185
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/ABAB:82:3:185