Abstract
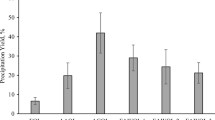
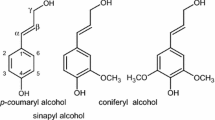
The oxidation of four lignins obtained by organosolv pulping of eucalyptus wood (Acetosolv-eucalyptus Acetosolv lignin [EAL]), sugarcanebagasse (Acetosolv-bagasse Acetosolv lignin [BAL] and in acetone/water/FeCl3-bagasse acetone/water lignin [BAWL]), and a softwood mixture (Organocell, Munich, Germany) was performed to obtain vanillin, vanillic acid, and oxidized lignin. Experiments were carried out in a cetic acid under oxygen flow using HBr, cobalt(II), and manganese(II) acetates as catalysts. After 10 h the total vanillin and vanillic acid yields were BAL 0.05 mmol, EAL 0.38 mmol, BAWL 0.45 mmol, and Organ ocell 0.84 mmol. Acetosolv lignins are crosslinked, which explains the lower yields in mononuclear products. The reaction volume (Δ V) of this reaction is −817 cm3/mol, obtained in experiments performed under oxygen pressure, showing the high influence of pressure on the oxidation. The major part of the, lignin stays in solution (oxidized lignin), which was analyzed by infrared spectroscopy, showing an increased in carbonyl and hydroxyl groups in comparison with the original lignin. The oxidized lignin can be used as chelating agent in the treatment of effluents containing heavy metals.
Similar content being viewed by others
References
Chang, H.-M. and Allan, G. G. (1971), in Lignins: Occurrence, Formation, Structure and Reaction, Sarkanen, K. V. and Ludwig, C. H., eds., Wiley-Interscience, New York, pp. 433–485.
Goheen, D. W. (1981), in Organic Chemicals from Biomass, Goldstein I. S., ed., CRC, Boca Raton, FL, pp. 143–161.
Hocking, M. B. (1997), J. Chem. Ed. 74, 1055–1059.
Kenny, J. (1997), PPI. 3, 23–27.
Raymond, A. Y. and Akhtar, M. (1998), Environmentally Friendly Technologies for the Pulp and Paper Industry, Wiley, New York.
Partenheimer, W. (1991), J. Mol. Cat. 67, 35–46.
Goncalves, A. R., Schuchardt, U., Meier, D., and Faix, O. (1993), Proceedings of the Third Brazilian Symposium on the Chemistry of Lignin and Other Wood Components, Belo Horizonte, Brazil, pp. 253–256.
Goncalves, A. R., Soto Oviedo, M. A., Urbano, M. P., Cotrim, A. R., and Silva, F. T. (1997), Proceedings of the Fifth Brazilian Symposium on the Chemistry of Lignins and Other Wood Components, Curitiba, Brazil, pp. 84–91.
Benar, P. and Schuchardt, U. F. (1994), Cell. Chem. Tech. 28, 435–444.
Benar, P. and Schuchardt, U. F. (1989), Proceedings of the First Bracilian Symposium on the Chemistry of Lignins and Other Wood Components, São Carlos, Brazil, pp. 186–192.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gonçalves, A.R., Schuchardt, U. Oxidation of organosolv lignins in acetic acid. Appl Biochem Biotechnol 77, 127–132 (1999). https://doi.org/10.1385/ABAB:77:1-3:127
Issue Date:
DOI: https://doi.org/10.1385/ABAB:77:1-3:127