Abstract
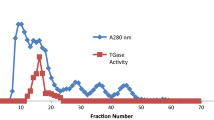
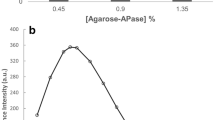
Aminoacylase (EC 3.5.1.14) was immobilized into DEAE-Sephadex A-25 by ion-exchange absorption for optical resolution of N-acyl-dl-alanine. The effects of pH, temperature, and Co2+ concentration on the activity of free and immobilized enzymes were in vestigated along with the operational and the thermal stability of the immobilized enzyme. The immobilized enzyme retained high catalytic activity. The optimum pH and temperature for the hydrolysis of N-acyl-l-alanine in the dl-isomer mixture were 8.0 and 65°C, respectively. Co2+ was an activator for the immobilized enzyme in a similarroleas for the free enzyme. Nosignificant loss of activity was observed for at least 300 h of continuous operation. The yield of l-alanine was about 70% of the theoretical yield. The immobilized aminoacylase column decayed over a very long period of operation, but could be completely reactivated by regeneration.
Similar content being viewed by others
References
Mattiasson, B. (1983), in Immobilized Cell and Organelles, vol. 1, Mattiason, B., ed., CRC Press, Boca Raton, FL, pp. 3–5.
Chibata, I., Tosa, T., Sato, T., and Mori, T. (1976), Methods Enzymol. 44 (51), 746–759.
Lee, P. M., Lee, K. H., and Siaw, Y. S. (1993), J. Chem. Tech. Biotechnol. 58, 65–70.
Lee, P. M., Lee, K. H., and Siaw, Y. S. (1992), J. Chem. Tech. Biotechnol. 54, 375–382.
Lee, P. M., Lee, K. H., and Siaw, Y. S. (1993), J. Chem. Tech. Biotechnol. 57, 27–32.
Tsai, Y. C., Lin, C. S., Tseng, T. H., Lee, T., and Wang, Y. J. (1992), Enzyme Microb. Technol. 14, 384–389.
Birnbaum, S. M. (1955), Methods Enzymol. 2, 115–119.
Lowry, O. H., Rosebrough, N. T., Farr, A. L., and Randall, R. J. (1951), J. Biol. Chem. 193, 265–275.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, HJ., Bai, JH., Liu, DS. et al. Preparation and properties of immobilized pig kidney aminoa cylase and optical resolution of N-acyl-dl-alanine. Appl Biochem Biotechnol 76, 183–191 (1999). https://doi.org/10.1385/ABAB:76:3:183
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/ABAB:76:3:183