Abstract
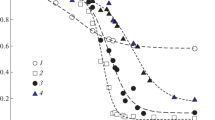
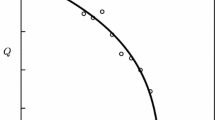
Temperature dependencies of kinetic and equilibrium parameters of urea hydrolysis catalyzed by native urease and the urease immobilized in a thermosensitive poly-N-isopropylacrylamide gel have been studied. The swelling ratio of the collapsed urease-containing gel is shown to increase in the presence of urea. Below a lower critical solution temperature (LCST) of the polymer, the immobilized u reaseactually has thesame catalytic properties as the native enzyme. At temperatures above LCST, the observed catalytic activity of the immobilized enzyme depends chiefly not only on the thermoreversible matrix state, but also on gel water content.
Similar content being viewed by others
Abbreviations
- E a :
-
activation energy, kJ/mol
- K m :
-
Michaelis constant, M
- V :
-
reaction rate, mM/min
- V max :
-
maximum reaction rate, mM/min
- V max.opf :
-
maximum reaction rate in a pH optimum of enzyme activity, mM/min
- T :
-
temperature, K
- t :
-
temperatue, °C
- ([NH2)2CO):
-
urea concentration, M
References
Hoffman, A. S. (1990), Polymerpreprints 31, pp. 220,221.
Ire, M. (1993), in Responsive Gels: Volume Transitions II, Dusek, K., ed., Springer-Verlag, Berlin, pp. 50–65.
Kokufuta, E. (1993), in Responsive Gels: Volume Transitions II, Dusek, K., ed., Springer-Verlag, Berlin, pp. 157–177.
Galayev, I. Yu. (1994), Biochemistry (Moscow) 59, 1478–1482.
Tanaka, T. (1978), Phys. Rev. Lett. 40, 820–823.
Bae, Y. H., Okano, T., and Kim, S. W. (1990), J. Polym. Sci., Part B: Polym. Phys. 28, 923–936.
Dong, L. C. and Hoffman, A. S. (1986), J. Controlled Release, 4, 223–227.
Park, T. G. and Hoffman, A. S. (1990), J. Biomed. Mat. Res. 24, 21–38.
Eremeev, N. L., Sigolaeva, L. V., and Kazanskaya, N. F. (1992), Vestn. MGU. Ser. Khim. 33, 511–515.
Kokufuta, E., Ogane, O., Iehijo H., Watanabe, S., and Hirasa, O. (1992), J. Chem. Soc. Chem. Commun. 5, 416–418.
Sigolaeva, L. V., Eremeev, N. L., and Kazanskaya, N. F. (1993), Biotckhnologiya 5, 36–39.
Sigolaeva, L. V., Ermeeev, N. L., and Kazanskaya, N. F. (1994), Bioorg. Khim. 20, 268–273.
Ermeev, N. L., Sigolaeva, L. V., Simakov, P. A., and Kazanskaya, N. F. (1995), Biochemistry (Moscovo) 60, 991–999.
Kokufuta, E., Zhang, Y.-Q., Ilmain, F., and Tanaka, T. (1991), Polym. Prep. Jpn. 40, 4204–4210.
Park, T. G. and Hoffman, A. S. (1988), Appl. Biochem. Biotechnol. 19, 1–9.
Raison, J. K., Lyons, J. M., and Tomson, W. W. (1971), Arch. Biochem. Biophys. 142, 83–90.
Watson, K., Bertoli, E., and Griffiths, D. E. (1975), Biochem. J. 146, 401–407.
Mamada, A., Tanaka, T., Kungwatchakun, D., and Irie, M. (1990), Macromolecules 23, 1517–1519.
Kokufuta, E., Zhang, Y.-Q., and Tanaka, T. (1991), Nature 351, 302–304.
Plaut, H. and Ritter, J. J. (1951), J. Am. Chem. Soc. 83, 4076,4077.
Dixon, M. and Webb, E. (1982), Enzymes (Russian translation), vol. 1, Mir, Moscow.
Lynn, K. R. (1967), Biochim. Biophys. Acta 146, 205–218.
Huang, T.-C. and Chen, D.-H. (1992), J. Chem. Tech. Biotechnol. 55, 45–51.
Huang, T.-C. and Chen, D.-H. (1991), J. Chem. Tech. Biotechnol. 52, 443,444.
Qin, Y. and Cabral, J. M. S. (1994), Appl. Biochem. Biotechnol. 49, 217–240.
Martins, M. B. F., Cruz, M. E. M., Cabral, J. M. S., and Kennedy, J. F. (1987), J. Chem. Tech. Biotechnol. 39, 201–206.
Jansen, M. and Blume, A. (1995), Biophys. J. 68, 997–1008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eremeev, N.L., Kukhtin, A.V., Belyaeva, E.A. et al. Effect of thermosensitive matrix-phase transition on urease-catalyzed urea hydrolysis. Appl Biochem Biotechnol 76, 45–55 (1999). https://doi.org/10.1385/ABAB:76:1:45
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/ABAB:76:1:45