Abstract
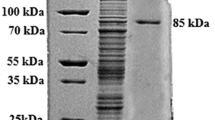
For the first time, a polygalacturonase from the culture broth of Tetracoccosporium sp. was isolated and incubated at 30°C in an orbital shaker at 160 rpm for 48h. The enzyme was purified by ammonium sulfate precipitation and two-step ion-exchange chromatography and had an apparent molecular mass of 36 kDa, as shown by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. Its optimum activity was at pH 4.3 and 40°C, and the K m and V max values of this enzyme (for polygalacturonic acid) were 3.23 mg/mL and 0.15 μmol/min, respectively. Ag+, Co2+, EDTA, Tween-20, Tween-80, and Triton X-100 stimulated polygalacturonase activity whereas Al3+, Ba2+, Ca2+, Fe2+, Fe3+, Ni2+, Mg2+, Mn2+, and SDS inhibited it. In addition, iodoacetamide and iodoacetic acid did not inhibit enzyme activity at a concentration of 1 mM, indicating that cysteine residues are not part of the catalytic site of polygalacturonase. We studied the kinetic properties and thermal inactivation of polygalacturonase. This enzyme exhibited a t 1/2 of 63 min at 60°C and its specific activity, turnover number, and catalytic efficiency were 6.17 U/mg, 113.64 min−1, and 35.18 mL/(min·mg), respectively. The activation energy (ΔE #) for heat inactivation was 5.341 kJ/mol, and the thermodynamic activation parameters ΔG #, ΔH #, and ΔS # were also calculated, revealing a potential application for the industry.
Similar content being viewed by others
References
Kaur, G., Kumar, S., and Satyanarayana, T. (2004), Bioresour. Technol. 94, 239–243.
Kirk, O., Borchert, T. V., and Fuglsang, C. C. (2002), Curr. Opin. Biotechnol. 13, 345–351.
Diana, D. (2001), Roum. Biotechnol. Lett 6(5), 397–402.
Soares, M. M. C. N., De Silva, R., and Gomes, E. (1999), Rev. Microbiol. 30, 299–303.
Kashyap, D. R., Vohra, P. K., Chopra, S., and Tewari, R. (2001), Bioresour. Technol. 77, 215–227.
Sakamoto, T., Bonnin, E., Quemener, B., and Thibault, J.-F. (2002), Biochim. Biophys. Acta 1572, 10–18.
Hoondal, G. S., Tiwari, R., and Dahiya, N. (2002), Appl. Microbiol. Biotechnol. 59, 409–418.
Ortega, N., de Diego, S., Perez-Mateos, M., and Busto, M. D. (2004), Food Chem. 88, 209–217.
Mohamed, S. A., Christensen, T. M. I. E., and Mikkelesen, J. D. (2003), Carbohydr. Res. 338, 515–524.
Ainsworth, G. C., Sparrow, F. K., and Sussman, F. S. (1973), The Fungi: An Advanced Treatise, Vol. IV. Pub. Academic, London.
Blandino, A., Dravillas, K., Cantero, D., Padiella, S. S., and Webb, C. (2001), Process Biochem. 37, 497–503.
Dartora, A. B., Bertolin, T. E., Bilibo, D., Silveria, M. M., and Costa, A. V. (2002), Z. Naturforsch. 57c, 666–670.
Miller, G. L. (1959), Anal. Chem. 31, 426–428.
Bonnin, E., Le Goff, A., Körner, R., et al. (2001), Biochim. Biophys. Acta 1526, 301–309.
Laemmli, U. K. (1970), Nature 227, 680–685.
Meril, C. R., Dunau, M. L., and Goldman, D. (1981), Anal. Biochem. 110, 201–207.
Beg, Q. K., Bushan, B., Kapoor, M., and Hoondal, G. S. (2000), J. Ind. Microbiol. 40, 974–977.
Huang, L. K., and Mahoney, R. R. (1999), J. Appl. Microbiol. 86, 145–156.
Acuña-Argüelles, M. E., Gutiérrez-Rojas, M., Viniegra-Gonzáles, G., and Favela-Torres, E. (1995), Appl. Microbiol. Technol. 43, 808–814.
Zheng, Z., and Shetty, K. (2000), Process Biochem. 35, 825–830.
Elegado, F. B., and Fujio, Y. (1993), J. Genet. Appl. Microbiol. 39, 409–418.
Ceci, L., and Lozano, J. (1998), Food Chem. 62(1/2), 237–241.
Rijssel, M., Gerwing, G. J., and Hansen, T. A. (1993), Appl. Environ. Microbiol. 59, 826–836.
Riou, C., Freyssinet, G., and Fever, M. (1992), Appl. Environ. Microbiol. 58, 578–583.
Blanco, P., Sieiro, C., Diaz, A., and Villa, T. G. (1994), Can. J. Microbiol. 40, 974–977.
Kapoor, M., Beg, Q.K., Bhushan, B., Dadhich, K. S., and Hoondal, G. S. (2000), Process Biochem. 36, 239–243.
Lourdes, M. D., Polizeli, T. M., Jorge, J. A., and Trenzi, H. F. (1991), J. Gen. Microbiol. 137, 1815–1823.
Sakiyama, C. C. H., Paola, E. M., Pereira, P. C., Borges, A. C., and Silva, D. O. (2001), Lett. Appl. Microbiol. 53, 117–221.
Fullbrook, P. D. (1996), in Industrial Enzymology, Godfrey, T. and West, S., eds., Macmillan, London, pp. 483–501.
Asther, M., and Meunier, J. C. (1990), Enzyme Microb. Technol. 12, 902–905.
Devi, N. A., and Rao, A. G. A. (1998), J. Agric. Food Chem. 46, 3540–3545.
Agblor, A., Henderson, H. M., and Madrid, F. J. (1994), Food Res. Int. 27, 321–326.
Naidu, G. S. N., and Panda, T. (2003), Biochem. Eng. J. 16, 57–67.
Chung, Y.-J., Cho, Y.-J., Chum, S.-S., and Choi, C. (1992), Korean Soc. Food Nutricional 21(2), 195–200.
Manjón, A., Iborra, J. L., Romero, C., and Canovas, M. (1992), Appl. Biochem. Biotechnol. 37, 19–31.
Busto, M. D., Owusu Apenten, R. K., Robinson, D. S., Wu, Z., Casey, R., and Hughes, R. K. (1999), Food Chem. 65, 323–329.
Brown, E. D. and Yada, R. Y. (1991), Biochim. Biophys. Acta 1076, 406–415.
Owusu, R. K. and Berthalon, N. (1993), Food Chem. 48, 231–235.
Stearn, A. E. (1949), Adv. Enzymol. 9, 25–74.
Ahern, T. J. and Klibanov, A. M. (1988), Methods Biochem. Anal., 33, 91–127.
Owusu, R. K., Makhzoum, A. M., and Knapp, J. S. (1992), Food Chem. 44, 261–268.
Dannenberg, F. and Kessier, H. I. G. (1988), J. Food Sci. 53, 258–263.
Grassin, C. and Fauquembergue, P. (1996), in Industrial Enzymology, Godfrey, T. and West, S., eds., Macmillan, London, pp. 226–264.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aminzadeh, S., Naderi-Manesh, H., Khajeh, K. et al. Purification, characterization, kinetic properties, and thermal behavior of extracellular polygalacturonase produced by filamentous fungus Tetracoccosporium sp. Appl Biochem Biotechnol 135, 193–208 (2006). https://doi.org/10.1385/ABAB:135:3:193
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/ABAB:135:3:193