Abstract
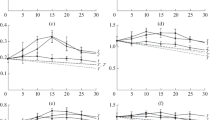
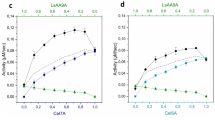
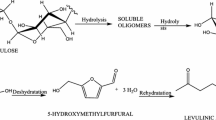
The integrated forms of the Michaelis-Menten equation assuming variable substrate (depletion) or constant substrate concentration were used to study the effect of the simultaneous presence of two exoglucanase Cel7A inhibitors (cellobiose and ethanol) on the kinetics of cellulose hydrolysis. The kinetic parameters obtained, assuming constant substrate (K m =21 mM, K ic =0.035 mM; K icl =1.5×1015mM; kcat=12 h−1) or assuming variable substrate (K m =16 mM, K ic =0.037 mM; K icl =5.8×1014 mM; kcat=9 h−1), showed a good similarity between these two alternative methodologies and pointed out that bothethanol and cellobiose are competitive inhibitors. Nevertheless, ethanol is a very weak inhibitor, as shown by the large value estimated for the kinetic constant K icl . In addition, assuming different concentrations of initial accessible substrate present in the reaction, both inhibition and velocity constants are at the same order of magnitude, which is consistent with the obtained values. The possibility of using this kind of methodology to determine kinetic constants in general kinetic studies is discussed, and several integrated equations of different Michaelis-Menten kinetic models are presented. Also examined is the possibility of determining inhibition constants without knowledge of the true accessible substrate concentration.
Similar content being viewed by others
Abbreviations
- A :
-
ethanol
- E :
-
free enzyme
- f 0.95 :
-
point of Fpa, pb (F distribution) curve with area 0.95 (to its right)
- I :
-
all inhibitors
- k cat :
-
catalytic constant (h−1)
- k ic :
-
competitive inhibition constant (mM) to cellobiose
- K icl :
-
competitive inhibition constant (mM) to ethanol
- K iu :
-
uncompetitive inhibition constant (mM) to cellobiose
- K iul :
-
uncompetitive inhibition constant (mM) to ethanol
- K m :
-
Michaelis constant (mM)
- n :
-
experimental points
- P :
-
reaction product (cellobiose)
- p A , p B :
-
parameters
- P 0 :
-
initial product
- Pt :
-
product at time t (min)
- S :
-
substrate
- t :
-
time (min)
- V max :
-
maximum velocity
- ω:
-
quotient used to test significance of improvement of different models interconvertible by addition or elimination of parameters by comparison of F-value
References
Holtzaple, M. T., Caram, H. S., and Humphrey, A. E. (1984), Biotechnol. Bioeng. 26, 753–757.
Pereira, A. N. (1987), PhD thesis, Purdue University, West Lafayette, IN.
Golovchenko, N. P., Kataeva, I. A., and Akimenko, V. K. (1992), Enzyme Microb. Technol. 14, 327–331.
Ramos, L. P., Breuil, C., and Saddler, J. N. (1993), Enzyme Microb. Technol. 15, 19–25.
Walker, L. P., Belair, C. D., Wilson, D. B., and Irwin, C. D. (1993), Biotechnol. Bioeng. 42, 1019–1028.
Gusakov, A. V. and Sinitsyn, A. P. (1992), Biotechnol. Bioeng. 40, 663–671.
Bezerra, R. M. F. and Dias, A. A. (2005), Appl. Biochem. Biotechnol. 126, 49–59.
Wu, Z. and Lee, Y. Y. (1997), Biotechnol. Lett. 19, 977–979.
Holtzaple, M., Cognata, M., Shu, Y., and Hendrickson, C. (1990), Biotechnol. Bioeng. 36, 275–287.
Ooshima, H., Ishitani, Y., and Harano, Y. (1985), Biotechnol. Bioeng. 27, 389–397.
Kennedy, J. F. and Cabral, J. M. S. (1987) (Enzyme Immobilization) in Biotechnology, Rehm, H. J. and Reed, G., eds., vol. 7a, Enzyme Technology, Kennedy, J. F., ed., VCH Verlagsgesellschaft, Weinheim, pp. 347–404.
Caldini, C., Bonomi, F., Pifferi, P. G., Lanzarini, G., and Galante, Y. M. (1994), Enzyme Microb. Technol. 16, 286–291.
Hsu, T.-A. and Tsao, G. T. (1979), Biotechnol. Bioeng. 21, 2235–2246.
Orsi, B. A. and Tipton, K. F. (1979), Methods Enzymol. 63, 159–183.
Duggleby, R. G. (2001), Methods 24(2), 168–174.
Markus, M., Hess, B., Ottaway, J. H., and Cornish-Bowden, A. (1976), FEBS Lett. 63(2), 225–230.
Foster, R. J. and Niemann, C. (1955), J. Am. Chem. Soc. 77, 1886–1892.
Philo, R. D. and Selwyn, M. J. (1973), Biochem. J. 135, 525–530.
Liao, F., Tian, K.-C., Yang, X., Zhou, Q.-X., Zeng, Z.-C., and Zuo, Y.-P. (2003), Anal. Bioanal. Chem. 375, 756–762.
Fernly, H. N. (1974), Eur. Biochem. J. 43, 377, 378.
Yun, S.-L. and Suelter, C. H. (1977), Biochim. Biophys. Acta 480, 1–13.
Bezerra, R. M. F. and Dias, A. A. (2004), Appl. Biochem. Biotechnol. 112, 173–184.
Beldman, G., Leeuwen, S.-V., Rombouts, F. M., and Voragen, F. G. J. (1985), Biochem. J. 146, 301–308.
Bezerra, R. M. F. (1999), Roum. Biotechnol. Lett. 4(4), 335–345.
Bezerra, R. M. (1995), PhD thesis, Universidade de Trás-os-Montes e Alto Douro, Vila Real, Portugal.
Bezerra, R. M. F. (1999), J. Med. Biochem. 3, 9–16.
Hsu, T.-H. (1979), PhD thesis, Purdue University, West Lafayete, IN.
Howell, J. A. and Stuck, J. D. (1975), Biotechnol. Bioeng. 17, 873–893.
Mannervick, B. (1982), Methods Enzymol. 87C, 370–391.
Kleman-Leyer, K. M. and Kirk, T. K. (1994), Appl. Environ. Microbiol. 60(8), 2839–2845.
Segel, I. H. (1975), Enzyme Kinetics, Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems, John Wiley & Sons, New York.
Bezerra, R. M. and Pereira, A. N. (1989), Ciência Biológica, Mol. Cell. Biol. (Portugal) 14(3/4), 71–79.
Kadam, K. L., Rydholm, E. C., and McMillan, J. D. (2004), Biotechnol. Prog. 20(3), 698–705.
Langmuir, I. (1916), J. Am. Chem. Soc. 38, 2221–2295.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bezerra, R.M.F., Dias, A.A., Fraga, I. et al. Simulaaneous ethanol and cellobiose inhibition of cellulose hydrolysis studied with integrated equations assuming constant or variable substrate concentration. Appl Biochem Biotechnol 134, 27–38 (2006). https://doi.org/10.1385/ABAB:134:1:27
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1385/ABAB:134:1:27