Abstract
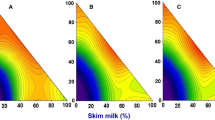
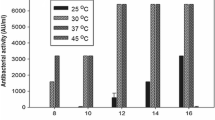
Nisin is a bacteriocin that inhibits the germination and growth of Gram-positive bacteria. With nisin expression related to growth conditions of Lactococcus lactis subsp. lactis, the effects of growth parameters, media components, and incubation time were studied to optimize expression. L. lactis ATCC 11454 was grown (100 rpm at 30°C for 36 h) in both M17 and MRS standard broth media (pH 6.0–7.0) supplemented with sucrose (1.0–12.5 g/L), potassium phosphate (0.13 g/L), asparagine (0.5 g/L), and sucrose (0.24 g/L), and diluted 1:1 with liquid nonfat milk. Liquid nonfat milk, undiluted, was also used as another medium (9% total solids, pH 6.5). Nisin production was assayed by agar diffusion using Lactobacillus sake ATCC 15521 (30°C for 24 h) as the sensitive test organism. The titers of nisin expressed and released in culture media were quantified and expressed in arbitrary units (AU/L of medium) and converted into known concentrations of “standard nisin” (Nisaplin®, g/L). The detection of nisin activity was <0.01 AU/L in M17 and MRS broths, and 7.5 AU/L in M17 with 0.14% sucrose or 0.13% other supplements, and the activity increased to 142.5 AU/L in M17 diluted with liquid nonfat milk (1:1). The 25% milk added to either 25% M17 or 25% MRS provided the highest levels of nisin assayed.
Similar content being viewed by others
References
Jung, G. (1991), Angew. Chem. Int. Ed. Engl. 30, 1051–1192.
Mattick, A. T. R. and Hirsh, A. (1944), Lancet 2, 3–7.
Buchman, G. W., Banerjee, S., and Hansen, J. N. (1988), J. BioL Chem. 263, 16,260–16,266.
De Vuyst and Vandamme, E. J. (1992), J. Gen. Microbiol. 138, 571–578.
Ray, B. (1992), in Food Biopreservatives of Microbial Origin, Ray, B. and Daeschel, M. A., eds., CRC Press, Boca Raton, Fl, pp. 1–23.
Vessoni Penna, T. C, Moraes, D. A., and Fajardo, D. N. (2002), J. Food Protect. 65, 419–422.
Turner, S. R., Love, R. M. and Lyons, K. M. (2004), Int. Endocrinol. J. 37, 664–671.
Sears, P. M., Smith, B. S. and Stewart, W. K. (1992), J. Dairy Sci. 75, 3185–3190.
Dubois, A. (1995), EID Dig. Dis. Div. 1(3), 79–88.
Sakamoto, I., Igarashi, M., and Kimura, K. (2001), J. Antimicrob. Chemother. 47, 709–710.
Stevens, K. A., Sheldon, B. W., Klapes, N. A., and Klaenhammer, T. R. (1991), J. Food Protect. 55,763–7766.
De Vuyst, L. and Vandamme, E. J. (1994), in Bacteriocins of Lactic Acid Bacteria, De Vuyst, L. and Vandamme, E. J., eds., Chapman & Hall, Glasgow, pp. 1–12.
Thomas, L. V. and Delves-Broughton, J. (2001), Res. Adv. Food Sci. 2, 11–22.
Thomas, L. V., Ingram, R. E., Bevis, H. E., Davies, A., Milne, C. F., and Delves-Broughton, J. (2002), J. Food. Protect. 65 (10), 1580–1585.
Wirjantoro, T.I. Lewis, M. J., Grandison, A. S., Williams, G. C., and Delves-Broughton, J. (2001), J. Food Protect. 64(2), 213–219.
Wandling, L. R., Sheldon, B. W., and Foegeding, P. M. (1999), J. Food. Protect. 65(5), 492–498.
Biswas, S. R., Ray, P., Johnson, M. C., and Ray, B. (1991), J. Appl. Environ. Microbiol. 57, 1265–1267.
Daba, H., Lacroix, C., Huang, J., and Simard, R. E. (1993), Appl. Microbiol. Biotechnol 39, 166–173.
Parente, E., Ricciardi, A., and Addario, G. (1994), Appl. Microbiol. Biotechnol. 41, 388–394.
Parente, E. and Ricciardi, A. (1994), Lett. Appl. Microbiol. 19, 12–15.
Yang, R. and Ray, B. (1994), Food Microbiol. 11, 281–291.
ten Brink, B., Minekus, M., van der Vossen, J. M, Leer, R. J., and Huis in’t Veld, J. H. (1994), J. Appl. Bacteriol. 77, 140–148.
Cheigh, C.-I., Choi, H.-J., Park, H., Kim, S.-B., Kook, M.-C Kim, T.-S., Hwang, J. K., and Pyun, Y. R. (2002), J. Biotechnol, 95, 225–235.
Kim, W. S., Hall, R. J., and Dunn, N. W. (1997), Appl. Microbiol BiotechnoL 48, 449–453.
Chandrapatti, S. and O’Sullivan, D. J. (1998), J. Biotechnol. 63, 229–233.
MacGroary, J. A. and Reid, G. (1988), Can. J. Microbiol. 39, 974–978.
Toba, T., Samant, S. K., Toshiota, E and Itoh, T. (1991), Lett. Appl. Microbiol. 13, 281–286.
Vessoni Penna, T.C. and Moraes, D. A. (2002), Appl. Biochem. Biotechnol. 98–100, 775–789.
Kim, W. S., Hall, R. J., and Dunn, N. W. (1998), Appl Microbiol Biotechnol. 50, 429–433.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Penna, T.C.V., Jozala, A.F., De Lencastre Novaes, L.C. et al. Production of nisin by Lactococcus lactis in media with skimmed milk. Appl Biochem Biotechnol 122, 619–637 (2005). https://doi.org/10.1385/ABAB:122:1-3:0619
Issue Date:
DOI: https://doi.org/10.1385/ABAB:122:1-3:0619