Abstract
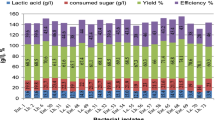
The performance of immobilized Bifidobacterium longum in sodium alginate beads and on a spiral-sheet bioreactor for the production of lactic acid from cheese whey was evaluated. Lactose utilization and lactic acid yield of B. longum were compared with those of Lactobacillus helveticus. B. longum immobilized in sodium alginate beads showed better performance in lactose utilization and lactic acid yield than L. helveticus. In the spiral-sheet bioreactor, a lactose conversion ratio of 79% and lactic acid yield of 0.84 g of lactic acid/g of lactose utilized were obtained during the first run with the immobilized L. helveticus. A lactose conversion ratio of 69% and lactic acid yield of 0.51 g of lactic acid/g of lactose utilized were obtained during the first run with immobilized B. longum in the spiral-sheet bioreactor. In producing lactic acid L. helveticus performed better when using the Spiral Sheet Bioreactor and B. longum showed better performance with gel bead immobilization. Because B. longum is a very promising new bacterium for lactic acid production from cheese whey, its optimum fermentation conditions such as pH and metabolic pathway need to be studied further. The ultrafiltration tests have shown that 94% of the cell and cheese whey proteins were retained by membranes with a mol wt cutoff of 5 and 20 KDa.
Similar content being viewed by others
References
Siso, M. I. G. (1996), Bioresour. Technol. 57, 1–11.
Shahbazi, A., Salameh, M., and Ibrahim, A., (2005), Milchwissenschaft. 60, in press.
Tango, M. S. A. and Ghaly, A. E. (2002), Appl. Microbiol. Biotechnol. 58, 712–720.
Roy, D., Goulet, J., and LeDuy, A. (1986), Appl. Microbiol. Biotechnol. 24, 206–213.
Bruno-Barcena, J. M., Ragout, A. L., Cordoba, P. R., and Sineriz, F. (1999), Appl. Microbiol. Biotechnol. 51, 316–324.
Roukas, T. and Kotzekidou, P. (1998), Enzyme Microbiol. Technol. 22, 199–204.
Gomes, A. M. P. and Malcata, F. X. (1999), Trends Food Sci. Technol. 10, 139–157.
Doleyres, Y., Paquin, C., LeRoy, M., and Lacroix, C. (2002), Appl. Microbiol. Biotechnol. 60, 168–173.
Song, S. H., Kim, T. B., Oh, H. I., and Oh, D. K. (2003), World J. Microbiol. Biotechnol. 19, 721–731.
Senthuran, A., Senthuran, V., Mattiasson, B., and Kaul, R. (1996), Biotechnol. Bioeng. 55(6), 841–853.
Mostafa, N. A. (1995), Energy Convers. Mgmt. 37(3), 253–260.
Senthuran, A., Senthuran, V., Hatti-Kaul, R., and Mattiasson, B. (1999), J. Biotechnol. 73, 61–70.
Guoqiang, D., Kaul, R., and Mattiasson, B. (1992), Appl. Microbiol. Biotechnol. 36, 309–314.
Corton, E., Piuri, M., Battaglini, F and Ruzal, S. M. (2000), Biotechnol. Prog. 16(1), 59–63.
Persson, A., Jonsson, A., and Zacchi, G. (2001), Biotechnol. Bioeng. 72(3), 269–277.
Mehaia, M. A. and Cheryan, M. (1986), Enzyme Microbiol. Technol. 8, 289–292.
Jeantet, R., Maubois, J. L., and Boyaval, P. (1996), Enzyme Microbiol. Technol. 19, 614–619.
Hjorleifsdottir, S., Holst, O., and Mattiasson, B. (1991), Bioprocess. Eng. 6, 29–34.
Foss Tecator. (1999). Application Note. The Determination of Nitrogen According to Kjeldahl using Block Digestion and Steam Distillation. 2400/24600 Kjctcc® Auto Sampler System User Manual 10009165. Revl.l.
AOAC. (1995), Official Methods of Analysis of AOAC International, 17th ed. S587.A3: AOAC Official Method 991. 22. Arlington, VA: AOAC International.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shahbazi, A., Mims, M.R., Li, Y. et al. Lactic acid production from cheese whey by immobilized bacteria. Appl Biochem Biotechnol 122, 529–540 (2005). https://doi.org/10.1385/ABAB:122:1-3:0529
Issue Date:
DOI: https://doi.org/10.1385/ABAB:122:1-3:0529