Abstract
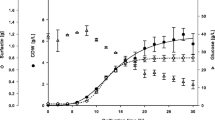
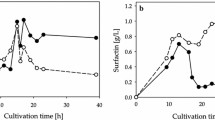
The biosurfactant surfactin has potential to aid in the recovery of energy resources (oil recovery) or subsurface organic contaminants (environmental remediation). However, high medium and purification costs limit its use in these high-volume applications. In previous work, we showed that surfactin could be produced from an inexpensive low-solids potato process effluent with minimal amendments or pretreatments. Previous research has also shown that surfactin can be both produced in Bacillus subtilis cultures and recovered by foam fractionation in an airlift reactor. Results using both purified potato starch and unamended low-solids potato process effluent as substrates for surfactin production indicate that the process is oxygen limited and that recalcitrant indigenous bacteria in the potato process effluent hamper continuous surfactin production. The research reported here features the use of a chemostat operated in batch mode for producing surfactin with concomitant use of antifoam to prevent surfactant loss. The antifoam did not interfere with surfactin recovery by acid precipitation or its efficacy. Initial trials took about 48 h to produce 0.9 g/L of surfactin from potato process effluent. Increasing the oxygen mass transfer by increasing the stirring speed and adding a baffle decreased production time to 12–24 h and produced about 0.6 g/L of surfactin from two different potato-processing facilities.
Similar content being viewed by others
References
Thompson, D. N., Fox, S. L., and Bala, G. A. (2000), Appl. Biochem. Biotechnol. 84–86, 917–930.
Thompson, D. N., Fox, S. L., and Bala, G. A. (2001), Appl. Biochem. Biotechnol. 91–93, 487–501.
Rosenberg, E. (1986), CRC Crit. Rev. Biotechnol. 3(3), 109–132.
Arima, K., Kakinuma, A., and Tamura, G. (1968), Biochem. Biophys. Res. Commun. 31, 488–494.
Cooper, D. G., McDonald, C. R., Duff, S. J. B., and Kosaric, N. (1981), Appl. Environ. Microbiol. 42, 408–412.
Besson, F. and Michel, G. (1992), Biotechnol. Lett. 14(11), 1013–1018.
Georgiou, G., Lin, S.-Y., and Sharma, M. M. (1992), Bio/Technology 10, 60–65.
Davis, D. A., Lynch, H. C., and Varley, J. (2001), Enzyme Microb. Technol. 28, 346–353.
Noah, K. S., Fox, S. L., Bruhn, D. F., Thompson, D. N., and Bala, G. A. (2002), Appl. Biochem. Biotechnol. 98–100, 803–813.
Gerhardt, P., Murray, R. G. E., Wood, W. A., and Kreig, N. R., eds. (1994), Methods for General and Molecular Bacteriology, Chapter 22 Chemical Analysis, L. Daniels, R. S. Hanson and J. A. Phillips. American Society for Microbiology, Washington, DC, pp. 518–519.
Herd, M. D., Lassahn, G. D., Thomas, C. P., Bala, G. A., and Eastman, S. L. (1992), Proceedings of the DOE Eighth Symposium on Enhanced Oil Recovery, Society of Petroleum Engineers/Department of Energy, April 22–24, Tulsa, OK. Vol#2. Paper SPE/DOE 24206, pp. 513–517.
Lin, S.-C. and Jiang, H.-J. (1997), Biotechnol. Techniques 11(6), 413–416.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noah, K.S., Bruhn, D.F. & Bala, G.A. Surfactin production from potato process effluent by Bacillus subtilis in a chemostat. Appl Biochem Biotechnol 122, 465–473 (2005). https://doi.org/10.1385/ABAB:122:1-3:0465
Issue Date:
DOI: https://doi.org/10.1385/ABAB:122:1-3:0465