Abstract
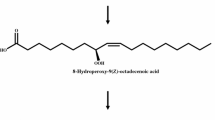
Potato tubers are shown to contain a unique lipoxygenase pathway to form 9-hydroperoxy-10,12-octadecadienoic acid (9-HPODE) from linoleic acid. Here, we report the metabolic pathway of 9-HPODE in the cytosolic fraction and the characterization of enzymes involved in the conversion of metabolites. The analysis of enzymatic reaction products at pH 5.5 revealed the formation of 9-keto-10,12-octadecadienoic acid, 9-hydroxy-10,12-octadecadienoic acid, 9,10-epoxy-11-hydroxy-12-octadecenoic acid, 9,10,13-trihydroxy-11-octadecenoic acid, and 9,12,13-trihydroxy-10-octadecenoic acid. The cytosolic enzymes were separated by anion-exchange chromatography into two fractions E1 and E2, having molecular masses of 66 and 54 kDa, respectively. The enzyme fraction E1 only produced 9-keto-10,12-octadecadienoic acid, whereas E2 formed other products. The enzyme E1 showed higher reactivity with 13- and 9-hydroperoxide of α-linolenic acid than 9-HPODE, but no reaction with hydroxy fatty acids. In contrast, the enzyme E2 showed the highest reactivity with 9-HPODE, followed by hydroperoxides of α-linolenic acid and arachidonic acid. We also evaluated the antibacterial activity of hydroxy fatty acids against Erwinia carotovora T-29, a bacterium infecting potato tubers. Growth of the bacteria was suppressed more potently with 9- or 13-hydroxy fatty acids than dihydroxy or trihydroxy fatty acids, suggesting a role for the metabolites in the resistance of bacterial infection.
Similar content being viewed by others
References
Briggs, W. R., Jones, R. L., and Walbot, V. (1991), Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 145–188.
Gardner, H. W. (1991), Biochim. Biophys. Acta 1084, 221–239.
Galliard, T. and Phillips, D. R. (1971), Biochem. J. 124, 431–438.
Mulliez, E., Leblanc, J.-P., Girerd, J.-J., Rigaud, M., and Chottard, J.-C. (1987), Biochim. Biophys. Acta 916, 13–23.
Shimizu, T., Radmark, O., and Samuelsson, B. (1984), Proc. Natl. Acad. Sci. USA 81, 689–693.
Bostock, R. M., Yamamoto, H., Choi, D., Ricker, K. E., and Ward, B. L. (1992), Plant Physiol. 100, 1448–1456.
Galliard, T. and Phillips, D. R. (1972), Biochem. J. 129, 743–753.
Galliard, T. and Phillips, D. R. (1973), Chem. Phys. Lipids 11, 173–180.
Gibian, M. J. and Vandenberg, P. (1987), Anal. Biochem. 163, 343–349.
Axelrod, B., Cheesbrough, T. M., and Laakso, S. (1981), Methods Enzymol. 71, 441–451.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951), J. Biol. Chem. 193, 265–275.
Hartree, E. F. (1972), Anal. Biochem. 48, 422–427.
Kenneth, B. W. and Saegeberth, K. A. (1957), J. Chem. Soc. 79, 2822–2824.
Royo, J., Cancanneyt, G., Perez, A. G., Sanz, C., Stormann, K., Rosahl, S., and Sanchez-Serrano, J. J. (1996), J. Biol. Chem. 271, 21,012–21,019.
Yokota, K., Lu, S., Takata, I., Kishimoto, A., Maeta, K., Nishimura, K., Nagaya, T., and Jisaka, M. (2003), in New Horizons in Biotechnology, Roussons, S., Soccol, C. R., Pandey, A., and Augur, C., eds., Kluwer Academic Publishers, Dordrecht, The Netherlands, pp. 199–214.
Galliard, T., Phillips, D. R., and Matthew, J. A. (1975), Biochim. Biophys. Acta 409, 157–171.
Galliard, T., Wardale, D. A., and Matthew, J. A. (1974), Biochem. J. 138, 23–31.
Itoh, A. and Howe, G. A. (2001), J. Biol. Chem. 276, 3620–3627.
Brash, A. R. and Song, W.-C. (1995), J. Lipid Mediat. Cell Signal 12, 275–282.
Song, W.-C., Funk, C. D., and Brash, A. R. (1993), Proc. Natl. Acad. Sci. USA 90, 8519–8523.
Hamberg, M. (2000), Lipids 35, 353–363.
Ohta, H., Shida, K., Peng, Y. L., Furusawa, A., Shisiyama, J., Aibara, S., and Morita, Y. (1990), Plant Cell Physiol. 31, 1117–1122.
Gardner, H. W., Dornbos, D. L., Jr., and Desjardins, A. E. (1986), J. Agric. Food Chem. 38, 1316–1320.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kimura, H., Yokota, K. Characterization of metabolic pathway of linoleic acid 9-hydroperoxide in cytosolic fraction of potato tubers and identification of reaction products. Appl Biochem Biotechnol 118, 115–132 (2004). https://doi.org/10.1385/ABAB:118:1-3:115
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/ABAB:118:1-3:115