Abstract
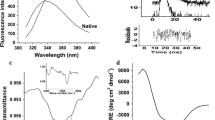
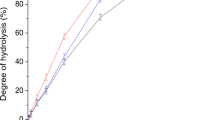
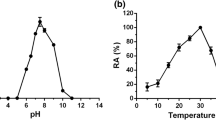
An intermediate from of α-amylase from Bacillus subtilis was prepared following a previously reported procedure. Stabilization of this protein structure by various additives and its interaction with alkyl-substituted Sepharose 4B (Sepharose-lipid), used to mimic the role of the alkyl chains of the phospholipid bilayer, were investigated. Exposure of hydrophobic clusters in the protein structure on denaturation was indicated by a greater affinity of the intermediate form for interaction with the alkyl chains on the matrix, as compared with the original native structure. Near- and far-ultraviolet circular dichroism studies supported loss of tertiary structure with retention of secondary structure, as expected from molten globular intermediate forms. Based on the results presented, we suggest that interaction of a protein in its native and nonnative forms with an alkyl-substituted matrix may provide useful information regarding its capacity for insertion into and translocation across the biologic membrane.
Similar content being viewed by others
References
Eilers, M. and Schatz, G. (1988), Cell 52(4), 481–483.
Simon, S. M., Peskin, C. S., and Oster, G. F. (1992), Proc. Natl. Acad. Sci. USA 89(9), 3770–3774.
Song, T. and Park, C. (1995), J. Mol. Biol. 253(2), 304–312.
Haddaoui, E., Leloup, L., Petit-Glatron, M. F., and Chambert, R. (1997), Eur. J. Biochem. 249, 505–509.
Laemmli, U. K. (1970), Nature 227(259), 680–685.
Hagele, E. O., Schaich, E., Rauscher, E., Lehmann, P., Burk, H., and Wahlefeld, A. W. (1982), Clin. Chem. 28(11), 2201–2205.
Bernfeld, P. (1955), in Methods in Enzymology, vol. 1, Collowick, S. P. and Kaplan, N. O., eds., Academic, New York, pp. 149–158.
Bradford, M. M. (1976), Anal. Biochem. 72, 248–254.
Nemat-Gorgani, M. and Karimian, K. (1982), Eur. J. Biochem. 123(3), 601–610.
Azari, F. and Nemat-Gorgani, M. (1999), Biotechnol. Bioeng. 62(2), 193–199.
Feller, G., Payan, F., Theys, F., Qian, M., Haser, R., and Gerday, C. (1994), Eur. J. Biochem. 222(2), 441–447.
Feller, G., d’Amico, D., and Gerday, C. (1999), Biochemistry 38(14), 4613–4619.
Machius, M., Declerck, N., Huber, R., and Wiegand, G. (1998), Structure 6(3), 281–292.
Hosseinkhani, S. and Nemat-Gorgani, M. (2003), Enzyme Microb. Technol. 33(2–3), 179–184.
Azari, F., Hosseinkhani, S., and Nemat-Gorgani, M. (2001), Appl. Biochem. Biotechnol. 94(3), 265–277.
Hosseinkhani, S., Moosavi-Movahedi, A. A., and Nemat-Gorgani, M. (2003), Appl. Biochem. Biotechnol. 110(3), 165–174.
Christensen, H. and Pain, R. H. (1991), Eur. Biophys. J. 19(5), 221–229.
Bychkova, V. E., Pain, R. H., and Ptitsyn, O. B. (1988), FEBS Lett. 238(2), 231–234.
Schwartz, M. P., Huang, S., and Matouschek, A. (1999), J. Biol. Chem. 274(18), 12,759–12,764.
Semisotnov, G. V., Rodionova, N. A., Razgulyaev, O. I., Uversky, V. N., Gripas’, A. F., and Gilmanshin, R. I. (1991), Biopolymers 31(1), 119–128.
Muzammil, S., Kumar, Y., and Tayyab, S. (1999), Eur. J. Biochem. 266(1), 26–32.
Koshiba, T., Yao, M., Kobashigawa, Y., Demura, M., Nakagawa, A., Tanaka, I., Kuwajima, K., and Nitta, K. (2000), Biochemistry 39(12), 3248–3257.
Matulis, D., Baumann, C. G., Bloomfield, V. A., and Lourien, R. E. (1999), Biopolymers 49(6), 451–458.
Rajaraman, K., Raman, B., and Rao, C. M. (1996), J. Biol. Chem. 271(44), 27,595–27,600.
Shin, I., Kreimer, D., Silman, I., and Weiner, L. (1997), Proc. Natl. Acad. Sci. USA 94(7), 2848–2852.
Van der Goot, F. G., Gonzalez-Manas, J. M., Lakey, J. H., and Pattus, F. (1991), Nature 354(6352), 408–410.
Nemat-Gorgani, M. and Karimian, K. (1983), Biotechnol. Bioeng. 25, 2617–2630.
Wong, S. L. (1995), Curr. Opin. Biotechnol. 6(5), 517–522.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karbalaei-Heidari, H.R., Habibi, A.E., Khajeh, K. et al. Interaction of an intermediate structure of Bacillus subtilis α-amylase with alkyl-substituted sepharose 4B. Appl Biochem Biotechnol 117, 123–132 (2004). https://doi.org/10.1385/ABAB:117:2:123
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/ABAB:117:2:123