Abstract
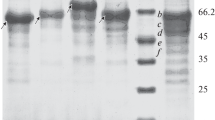
An extracellular exoinulinase was purified from the crude extract of Aspergillus fumigatus by ammonium sulfate precipitation, followed by successive chromatographies on DEAE-Sephacel, Sephacryl S-200, concanavalin A-linked amino-activated silica, and Sepharose 6B columns. The enzyme was purified 25-fold, and the specific activity of the purified enzyme was 171 IU/mg of protein. Gel filtration chromatography revealed a molecular weight of about 200 kDa, and native polyacrylamide gel electrophoresis (PAGE) showed an electrophoretic mobility corresponding to a molecular weight of about 176.5 kDa. Sodium dodecyl sulfate-PAGE analysis revealed three closely moving bands of about 66, 62.7, and 59.4 kDa, thus indicating the heterotrimeric nature of this enzyme. The purified enzyme appeared as a single band on isoelectric focusing, with a pI of about 8.8. The enzyme activity was maximum at pH 5.5 and was stable over a pH range of 4.0–9.5, and the optimum temperature for enzyme activity was 60°C. The purified enzyme retained 35.9 and 25.8% activities after 4 h at 50 and 55°C, respectively. The inulin hydrolysis activity was completely abolished with 1 mM Hg++, whereas EDTA inhibited about 63% activity. As compared to sucrose, stachyose, and raffinose, the purified enzyme had lower K m (0.25 mM) and higher V max (333.3 IU/mg) values for inulin.
Similar content being viewed by others
References
Chen, W. C. and Liu, C. H. (1996), Enzyme Microb. Technol. 1296, 153–160.
Elmer, G. W. (1986), JAMA 275, 870–876.
Hatcher, G. E. and Lambrecht, R. S. (1993), J. Dairy Sci. 76, 2485–2492.
Vandamme, E. J. and Derycke, D. G. (1983), Adv. Appl. Microbiol. 29, 139–176.
Kaur, A., Sharma, D., Harchand, R. K., Singh, P., Bhullar, S. S., and Kaur, A. (1999), Indian J. Microbiol. 39, 99–103.
Sharma, D., Kaur, A., Harchand, R. K., Singh, P., Bhullar, S. S., and Kaur, A. (1998), Indian J. Microbiol. 38, 235, 236.
Bradford, M. M. (1976), Anal. Biochem. 72, 248–254.
Nelson, N. (1944), J. Biol. Chem. 153, 375–380.
Singh, J., Kamboj, S. S., Sandhu, R. S., Shangary, S., and Kamboj, K. K. (1993), Phytochemistry 33, 979–983.
Laemmli, U. (1970), Nature 227, 680–685.
Gabriel, O. and Wang, S. F. (1969), Anal. Biochem. 27, 545–554.
Celis, J. E., Lauridsen, J. B., and Basse, B., eds. (1994), in Cell Biology: A Laboratory Handbook, 2nd ed., Academic, San Diego, pp. 305–313.
Trevelyan, W. E., Procter D. P., and Harrison, J. S. (1950), Nature 166, 444, 445.
Ettalibi, M. and Baratti, J. C. (1987), Agric. Biol. Chem. 54, 61–68.
Kochhar, A., Gupta, A. K., and Kaur, N. (1999), J. Sci. Food Agric. 79, 549–554.
Kochhar, A., Kaur, N., and Gupta, A. K. (1997), J. Sci. Ind. Res. 57, 184–187.
Arand, M., Golubev, A. M., Neto, J. R. B., et al. (2002), Biochem. J. 362, 131–135.
Nakamura, T., Kurokawa, T., Nakatsu, S., and Ueda, S. (1978), Nippon. Nogeikagaku. Kaishi. 52, 581–587.
Uhm, T. A. and Byun, S. M. (1987), Biotechnol. Lett. 9, 287–290.
Beluche, I., Guiraud, J. P., and Galzy, P. (1980), Folio Microbiol. 25, 32–39.
Guirad, J. P., Viard-Gaudin, C., and Galzy, P. (1980), Agric. Biol. Chem. 44, 245–252.
Derycke, D. G. and Vandamme, E. J. (1984), J. Chem. Tech. Biotechnol. 34, 45–51.
Pessoni, R. A. B., Figueiredo, R., and Braga, M. R. (1999), J. Appl. Microbiol. 87, 141–147.
Efstathiou, I., Reysset, G., and Truffaut, N. (1986), Appl. Microbiol. Biotechnol. 25, 143–149.
Kaur, N., Kaur, M., Gupta, A. K., and Singh, R. (1992), J. Chem. Tech. Biotechnol. 53, 279–284.
Hazaa, M. M. (1999), J. Union Arab. Biol. 8, 467–482.
Nakamura, T., Ogata, Y., Shitara, A., Nakamura, A., and Ohta, K. (1995), J. Ferment. Bioeng. 80, 164–169.
Nakamura, T., Shitara, A., Matsuda, S., Matsuo, T., Suiko, M., and Ohta, K. (1997), J. Ferment. Bioeng. 84, 313–318.
Nahm, B. H. and Byun, M. (1977), Korean Biochem. J. 10, 95–108.
Mukherjee, K. and Sengupta, S. (1987), Can. J. Microbiol. 33, 520–524.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gill, P.K., Manhas, R.K., Singh, J. et al. Purification and characterization of an exoinulinase from Aspergillus fumigatus . Appl Biochem Biotechnol 117, 19–32 (2004). https://doi.org/10.1385/ABAB:117:1:19
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/ABAB:117:1:19