Abstract
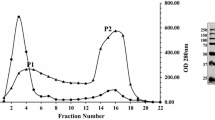
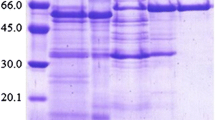
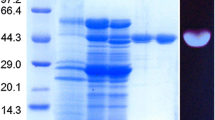
The filamentous fungus Sclerotinia sclerotiorum, grown on a xylose medium, was found to excrete one β-glucosidase (β-glu x). The enzyme was purified to apparent homogeneity by ammonium sulfate precipitation, gel filtration, anion-exchange chromatography, and high-performance liquid chromatography (HPLC) gel filtration chromatography. Its molecular mass was estimated to be 130 kDa by HPLC gel filtration and 60 kDa by sodium dodecyl sulfate polyacrylamide gel electrophoresis, suggesting that β-glu x may be a homodimer. For p-nitrophenyl β-d-glucopyranoside hydrolysis, apparent K m and V max values were found to be 0.09 mM and 193 U/mg, respectively, while optimum temperature and pH were 55–60°C and pH 5.0, respectively. β-Glu x was strongly inhibited by Fe2+ and activated about 35% by Ca2+. β-Glu x possesses strong transglucosylation activity in comparison with commercially available β-glucosidases. The production rate of total glucooligosaccharides (GOSs) from 30% cellobiose at 50°C and pH 5.0 for 6 h with 0.6 U/mL of enzyme preparation was 80 g/L. It reached 105 g/L under the same conditions when using cellobiose at 350 g/L (1.023 M). Finally, GOS structure was determined by mass spectrometry and 13C nuclear magnetic resonance spectroscopy.
Similar content being viewed by others
References
Lumsden, R. D. (1969), Phytopathology 59, 653–657.
Cooper, R. M. (1984), in Plant Disease: Infection, Damage and Loss, Wood, R. K. S. and Jelis, G. J., eds., Blackwell Scientific, Oxford, pp. 13–28.
Waksman, G. (1988), Biochem. Biophys. Acta 967, 82–86.
Martel, M. B., Léoublon, R., and Fvère, M. (1998), FEMS Microbiol. Lett. 158, 133–138.
Mayer, A. M. (1989), Phytochemistry 28, 311–317.
Guanta, Z. Y., Baynove, C. T., Tapiero, R. E., and Cordonnier, R. (1990), J. Agric. Food Chem. 38, 757–763.
Guguen, Y., Chemardin, P., Pien, S., Arnaud, A., and Galzy, P. (1997), J. Biotech. 55, 151–156.
Bhat, M. K. and Bhat, S. (1997), Biotechnol. Adv. 15, 583–620.
Kang, S. W., Ko, E. H., Lee, J. S., and Kim, S. W. (1999), Biotechnol. Lett. 21, 647–650.
Nilsson, K. G. I. (1988), Trends Biotechnol. 6, 256–264.
Vulfson, E. N., Rooma, P., and Law, B. A. (1990), Biotechnol. Lett. 12, 397–402.
Boons, G. J. (1996), Tetrahedron 52, 1095–1121.
Varki, A. (1993), Glycobiology 3, 97–130.
Monsan, P. and Paul, F. (1995), FEMS Microbiol. Rev. 16, 187–192.
Gama, F. M. and Mota, M. (1998), Carbohydr. Polym. 37, 279–281.
Palcic, M. M. (1999), Curr. Opin. Biotechnol. 10, 616–624.
Wymer, N. and Toone, E. J. (2000), Curr. Opin. Chem. Biol. 4, 110–119.
Flisch, S. L. (2000), Curr. Opin. Chem. Biol. 4, 619–625.
Prade, H., Mackenzie, L. F., and Withers, S. G. (1998), Carbohydr. Res. 305, 371–381.
Smaali, M. I., Gargouri, M., Limam, F., Fattouch, S., Maugard, T., Legoy, M. D., and Marzouki, N. (2003), Appl. Biochem. Biotechnol. 111, 23–40.
Miller, G. L. (1959), Anal. Chem. 31, 426–428.
Laemmli, U. K. (1970), Nature 227, 680–685.
Blum, H., Beier, H., and Gross, B. (1987), Electrophoresis 8, 93–99.
Bradford, M. M. (1976), Anal. Biochem. 72, 248–254.
Saha, B. C. and Bothast, R. J. (1996), Appl. Environ. Microbiol. 62, 3165–3170.
Hrmovà M., Petrakova, E., and Biely, P. (1991), J. Gen. Microbiol. 137, 541–547.
Watanabe, T., Sato, T., Yoshioka, S., and Kuwahara, M. (1992), Eur. J. Biochem. 209, 651–659.
Saha, B. C., Freer, S. N., and Bothast, R. J. (1994), Appl. Environ. Microbiol. 60, 3774–3780.
Filho, E. X. F. (1996), Can. J. Microbiol. 42, 1–5.
Ruttersmith, L. D. and Daniel, R. M. (1993), Biochim. Biophys. Acta 1156, 167–172.
Woodward, J. and Wiseman, A. (1982), Enzyme Microb. Technol. 4, 73–79.
Coughlan, M. P. (1985), Biotechnol. Genet. Eng. Rev. 3, 39–109.
Kengen, S. W. M., Leusink, E. J., Stams, A. J. M., and Zehnder, A. J. B. (1993), Eur. J. Biochem. 213, 305–312.
Yan, T. R. and Liau, J. C. (1998), Biotechnol. Lett. 20, 591–594.
Kono, H., Waelchli, M. R., Fujiwara, M., Erata, T., and Takai, M. (1999), Carbohydr. Res. 319, 29–37.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Issam, S.M., Mohamed, G., Dominique, L.M. et al. A β-glucosidase from Sclerotinia sclerotiorum . Appl Biochem Biotechnol 112, 63–77 (2004). https://doi.org/10.1385/ABAB:112:2:63
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/ABAB:112:2:63