Abstract
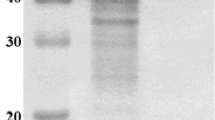
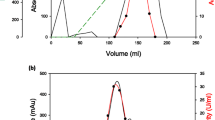
The saccharifying alkaline amylase and neopullulanase complex of Micrococcus halobius OR-1 hydrolyzes both α-(1,4)- and α-(1,6)-glycosidic linkages of different linear and branched polysaccharides. The following observations were made concerning the analysis of the coexpressed amylase and neopullulanase enzymes. Even though the enzymes were subjected to a rigorous purification protocol, the activities could not be separated, because both the enzymes were found to migrate in a single peak. By contrast, two independent bands of amylolytic activity at 70 kDa and pullulanolytic activity at 53 kDa were evident by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), reducing and nonreducing PAGE, and zymographic analysis on different polysaccharides. Preferential chemical modification of the enzyme and concomitant high-performance thin-layer chromatographic analyses of the saccharides liberated showed that amylase is sensitive to 1-(dimethylamino-propyl)-3-ethyl carbodiimide-HCl and cleaved α-(1,4) linkages of starch, amylose, and amylopectin producing predominantly maltotriose. On the other hand, formalin-sensitive neopullulanase acts on both α-(1,4) and α-(1,6) linkages of pullulan and starch with maltotriose and panose as major products. It is understood that neopullulanase exhibits dual activity and acts in synergy with amylase toward the hydrolysis of α-(1,4) linkages, thereby increasing the overall reaction rate; however, such a synergism is not seen in zymograms, in which the enzymes are physically separated during electrophoresis. It is presumed that SDS-protein intercalation dissociated the enzyme complex, without altering the individual active site architecture, with only the synergism lost. The optimum temperature and pH of amylase and neopullulanase were 60°C and 8.0, respectively. The enzymes were found stable in high alkaline pH for 24 h. Therefore, the saccharifying alkaline amylase and neopullulanase of M. halobius OR-1 evolved from tapioca cultivar shows a highly active and unique enzyme complex with several valuable biochemical features.
Similar content being viewed by others
References
Juge, N., Svensson, B., and Willianson, G. (1998), Appl. Microbiol. Biotechnol. 49, 385–392.
Cornelis, P. (1987), Microbiol. Sci. 4, 342, 343.
Norman, B. E. (1982), Starch-Starke 10, 340–346.
Plant, A. R., Clemend, R. M., Morgan, H. W., and Daniel, R. M. (1987), Biochem. J. 246, 537–541.
Kim, C. H. (1994), FEMS Microbiol. Lett. 116, 327–332.
Lee, Y. E., Jain, M. K., Lee, C., Lowe, S. E., and Zeikus, J. G. (1993), Int. J. Syst. Bacteriol. 43, 41–51.
Mathubala, S. P., Lowe, S. E., Podkovyrov, S. M., and Zeikus, J. G. (1993), J. Biol. Chem. 268, 16,332–16,344.
Sata, H., Umeda, M., Kim, C. H., Taniguchi, H., and Maruyama, Y. (1989), Biochim. Biophys. Acta 991, 388–394.
Kuriki, T., Okada, S., and Imanaka, T. (1988), J. Bacteriol. 170, 1554–1559.
Imanaka, T. and Kuriki, T. (1989), J. Bacteriol. 171, 369–374.
Lee, C., Saha, B. C., and Zeikus, J. G. (1990), Appl. Environ. Microbiol. 56, 2895–2901.
RajDevi, K. P. and Yogeeswaran, G. (1999), World J. Microbiol. Biotechnol. 15(2), 223–227.
Somogyi, M. (1952), J. Biol. Chem. 195, 19–23.
Bradford, M. M. (1976), Anal. Biochem. 72, 248–254.
Rylatt, D. B. and Parish, C. R. (1982), Anal. Biochem. 121, 213, 214.
Gupta, K. C., Sahni, M. K., and Rathore, B. S. (1989), J. Chromatogr. 169, 183.
Sundberg, L. and Porath, J. (1974), J. Chromatogr. 40, 87–98.
Laemmli, U. K. (1970), Nature 227, 680–685.
Fairbanks, G., Steck, T. L., and Wallach, D. F. H. (1971), Biochemistry 10, 2606–2617.
Mathubala, P. S. and Zeikus, J. G. (1993), Appl. Microbiol. Biotechnol. 487–493.
Carraway, K. L. and Koshland, D. E. (1972), Methods Enzymol. 25, 616–623.
Boopathy, R. and Balasubramanian, A. S. (1985), Eur. J. Biochem. 151, 351–360.
Itkor, P., Tsukagoshi, N., and Udaka, S. (1989), J. Ferment. Bioeng. 68, 247–251.
Ara, K., Igarashi, K., Saeki, K., and Ito, S. (1995), Biosci. Biotechnol. Biochem. 59, 662–666.
Bender, H. and Wallenfels, K. (1966), Methods Enzymol. 8, 555–562.
Takata, H., Kuriki, T., Okada, S., Takesada, Y., Iizuka, M., Minamiura, N., and Imanaka, T. (1992), J. Biol. Chem. 267, 18,447–18,452.
Kim, C. H., Choi, H. I., and Lee, D. S. (1993) J. Ind. Microbiol. 12, 48–57.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rajdevi, K.P., Yogeeswaran, G. Cooperativity and substrate specificity of an alkaline amylase and neopullulanase complex of Micrococcus halobius OR-1 . Appl Biochem Biotechnol 90, 233–249 (2001). https://doi.org/10.1385/ABAB:90:3:233
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/ABAB:90:3:233