Abstract
Phenyl ethyl alcohol is a compound that occurs naturally in flower petals and in many common beverages, such as beer. Desire for the floral, rose-like notes imparted by phenyl ethyl alcohol has created a unique niche for this chemical in flavor and fragrance industries. Phenyl ethyl alcohol can be produced by Saccharomyces cerevisiae via bioconversion. Often this method of production results in extremely low yields, thus placing a great deal of importance on recovery and purification of the valuable metabolite.
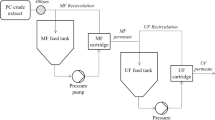
To determine the best method for recovering the chemical, a primary recovery step and a secondary recovery step were developed. The primary recovery step consisted of comparing dead-end filtration with crossflow ultrafiltration. Crossflow ultrafiltration was ultimately selected to filter the fermentation broth because of its high flow rates and low affinity for the product. The secondary recovery step consisted of a comparison of liquid-liquid extraction and hydrophobic resin recovery. The hydrophobic resin was selected because of its higher rate of recovery and a higher purity than the liquid-liquid extraction, the current practice of Brown-Forman.
Similar content being viewed by others
References
Gabelman, A. (ed.) (1984), Bioprocess Production of Flavors, Fragrance and Color Ingredients, Wiley, New York, pp. 1–17.
Häuser, A. and Münch, T. (1997), ASM News 63, 551.
Fukuda, K., Watanabe, M., Asano, K., Ueda, H., and Ohta, S. (1989), Agricult. Biol. Chem. 54, 269–271.
Patterson, R. L. S., Charlwood, B. V., MacLeod, G., and Williams, A. A. (1992), Bioformation of Flavours, The Royal Society of Chemistry Press, Cambridge, UK.
Priddy, D. L. (1993), Interoffice correspondence provided by Brown-Forman Corporation, Louisville, KY.
Atkinson, B. and Mauituna, F. (1991), Biochemical Engineering and Biotechnology Handbook, Stockton, New York, pp. 900–1021.
Priddy, D. L. (1995), Interoffice correspondence provided by Brown-Forman Corporation, Louisville, KY.
Priddy, D. L. (1996), Interoffice correspondence provided by Brown-Forman Corporation, Louisville, KY.
Catalog Handbook of Fine Chemicals, (1996–1997), Aldrich, Milwaukee, WI.
Gabelman, A. (ed.) (1984), Bioprocess Production of Flavors, Fragrance and Color Ingredients, Wiley, New York, pp. 34–58.
Priddy, D. L. (1998), Brown-Forman Corporation, personal communication.
Priddy, D. L. (1998), Brown-Forman Corporation, personal communication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Priddy, S.A., Hanley, T.R. & Effler, W.T. Separation optimization for the recovery of phenyl ethyl alcohol. Appl Biochem Biotechnol 78, 473–484 (1999). https://doi.org/10.1385/ABAB:78:1-3:473
Issue Date:
DOI: https://doi.org/10.1385/ABAB:78:1-3:473