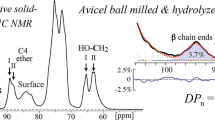
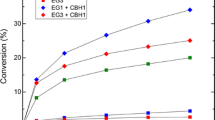
Abstract
Methods for the quantification of total and accessible reducing ends on traditional cellulose substrates have been evaluated because of their relevance to enzyme-catalyzed cellulose saccharification. For example, quantification of accessible reducing ends is likely to be the most direct measure of substrate concentration for the exo-acting, reducing end-preferring cellobiohydrolases. Two colorimetric assays (dinitrosalicylic acid [DNS] and bicinchoninic acid [BCA] assay) and a radioisotope approach (NaB3H4 labeling) were evaluated for this application. Cellulose substrates included microcrystalline celluloses, bacterial celluloses, and filter paper. Estimates of the number of reducing ends per unit mass cellulose were found to be dependent on the assay system (i.e. the DNS and BCA assays gave strikingly different results). DNS-based values were several-fold higher than those obtained using the BCA assay, with fold-differences being substrate specific. Sodium borohydride reduction of celluloses, using cold or radiolabeled reagent under relatively mild conditions, was used to assess the number of surface (solvent-accessible) reducing ends. The results indicate that 30–40% of the reducing ends on traditional cellulose substrates are not solvent accessible; that is, they are buried in the interior of cellulose structures and thus not available to exo-acting enzymes.
Similar content being viewed by others
References
Gilkes, N. R., Henrissat, B., Kilburn, D. G., Miller, R. C. Jr., and Warren, R. A. J. (1991), Microbiol. Rev. 55, 303–315.
Henrissat, B. (1991), Biochem. J. 280, 309–316.
Goyal, A., Ghosh, B., and Eveleigh, D. (1991), Bioresour. Technol. 36, 37–50.
Tomme, P., Warren, R. A. J., and Gilkes, N. R. (1995), Adv. Microb. Physiol. 37, 1–81.
Davies, G. and Henrissat, B. (1995), Structure 3, 853–859.
Henrissat, B., Callebaut, I., Faberega, S., Lehn, P., Mormon, J. P., and Davies, G. (1995), Proc. Natl. Acad. Sci. USA 92, 7090–7094.
Teeri, T. T. (1997), Trends Biotechnol. 160, 155–195.
Schulein, M. (2000), Biochimg Biophys Acta 1543, 239–252.
Henrissat, B. and Davies, G. (1997), Curr. Opin. Struct. Biol. 7, 637–644.
Divne, C., Stahlberg, J., Teeri, T. T., and Jones, T. A. (1998), J. Mol. Biol. 275, 309–325.
Zou, J. Y., Kleywegt, G. J., Stahlberg, J., Driguez, H., Nerinckx, W., Claeyssens, M., Koivula, A., Teeri, T. T., and Jones, T. A. (1999), Structure 7, 1035–1045.
Koivula, A., Laura, R., Gerd, W., et al. (2002), J. Am. Chem. Soc. 124, 10,015–10,024.
White, A. and Rose, D. R. (1997), Curr. Opin. Struct. Biol. 7, 645–651.
Bayer, E. A., Chanzy, H., Lamed, R., and Shoham, Y. (1998), Curr. Opin. Struct. Biol. 8, 548–557.
Medve J., Karlsson, J., Lee, D., and Tjerneld, F. (1998), Biotechnol. Bioeng. 59, 621–634.
Nidetzky, B., Steiner, W., and Claeyssens, M. (1995), in Enzymatic Degradation of Insoluble Carbohydrate. Saddler, J. N. and Penner, M. H., eds., American Chemical Society, Washington, DC, pp. 90–112.
Gama, F. M., Teixeira, J. A., and Mota, M. (1994), Biotechnol. Bioeng. 43, 381–387.
Lin, J. K., Ladisch, M. R., Patterson, J. A., and Noller, C. H. (1987), Biotechnol. Bioeng. 24, 976–981.
Neuman, R. P. and Walker, L. P. (1992), Biotechnol. Bioeng. 40, 218–225.
Walker, L. P., Wilson, D. B., Irwin, D. C., McQuire, C., and Price, M. (1992), Biotechnol. Bioeng. 40, 1019–1026.
Grethlein, H. E. (1978), Biotechnol. Bioeng. 20, 503–525.
Isogai, A. and Atalla, R. H. (1991), J. Polyma Sci.: Part A. Polyma Chem. 29, 113–119.
Ståhlberg, J., Johasson, G., and Peterson, G. (1993), Biochim. Biophys. Acta 1157, 107–113.
Hestrin, S. (1963), Methods Carbohydr. Chem. 3, 4–9.
Gilkes, N. R., Jervis, E., Henrissat, B., Tekant, B., Miller, R. C. Jr., Warren, R. A. J., and Kilburn, D. G. (1992), J. Biol. Chem. 267, 6743–6749.
Ghose, T. K. (1987), Pure Appl. Chem. 59, 257–268.
Nelson, N. J. (1944), J. Biol. Chem. 153, 375–380.
Robyt, J. F. and Whelan, W. J. (1972), Anal. Biochem. 45, 510–516.
Lever, M. (1972), Anal. Biochem. 47, 273–279.
Garcia, E., Johnston, D., Whitaker, J., and Shoemaker, S. (1993), J. Food Biochem. 17, 135–145.
Johnston, D. B., Shoemaker, S. P., Smith, G. M., and Whitaker, J. R. (1998), J. Food Biochem. 22, 301–319.
Irwin, D. C., Spezio, M., Walker, L. P, and Wilson, D. B. (1993), Biotechnol. Bioeng. 42, 1002–1013.
Conrad, H. E., Bamburg, J. R., Epley, J. D., and Kindt, T. J. (1966), Biochemistry 5, 2808–2817.
Boraston, A. B., Creagh, A. L., Alam, M. M., Kormos, J. M., Tomme, P., Haynes, C. A., Warren, R. A. J., and Kilburn, D. G. (2001), Biochemistry 40, 6240–6247.
Kruus, K., Wang, W. K., Ching, J. and Wu, J. H. D. (1995), J. Bacteriol. 177, 1641–1644.
Irwin, D., Shin, D. H., Zhang, S., Barr, B. K., Sakon, J., Karplus, P. A., and Wilson, D. B. (1998), J. Bacteriol. 180, 1709–1714.
Kim, E., Irwin, D. C., Walker, L. P., and Wilson, D. B. (1998), Biotechnol. Bioeng. 58, 494–501.
Zhang, S., Wolfgang, D. E., and Wilson, D. B. (1999), Biotechnol. Bioeng. 66, 35–41.
Zhang, S., Barrt, B. K., and Wilson, D. B. (2000), Eur. J. Biochem. 267, 244–252.
Zhang, S., Irwin, D. C., and Wilson, D. B. (2000), Eur. J. Biochem. 267, 3101–3115.
Kulshreshtha, A. K. and Dweltz, N. E. (1973), J. Polym. Sci. Polym. Phys. Ed. 11, 487–497.
Brooks, R. D. and Thompson, N. S. (1966), TAPPI. J. 49, 362–366.
Liaw, E. T. (1994), PhD thesis, Oregon State University, Corvallis, OR.
Stalbrånd, H., Mansfield, S. D., Saddler, J. N., Kilburn, D. G., Warren, R. A. J., and Gilkes, N. R. (1998), Appl. Environ. Microbiol. 64, 2374–2379.
Michell, A. J. (1993), Cell. Chem. Technol. 27, 3–15.
Fan, L. T., Lee, Y. H., and David, H. B. (1980), Biotechnol. Bioeng. 22, 177–199.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kongruang, S., Han, M.J., Breton, C.I.G. et al. Quantitative analysis of cellulose-reducing ends. Appl Biochem Biotechnol 113, 213–231 (2004). https://doi.org/10.1385/ABAB:113:1-3:213
Issue Date:
DOI: https://doi.org/10.1385/ABAB:113:1-3:213