Abstract
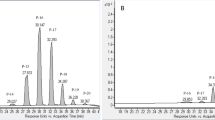
The intact plant parts and genetically modified hairy root clone #TpA6 of Tagetes patula were extracted with supercritical fluid CO2 extraction (SFE) and a conventional solvent extraction. SFE optimization included the variation of fluid CO2 pressure, dynamic time, and the addition of methanol modifier co-solvent. The four characteristic thiophene metabolites, 5-(3-buten-1-ynyl)-2,2′-bithienyl (BBT), 2,2′:5′,2″-terthiophene (α-T), 5-(4-acetoxy-1-butynyl)-2,2′-bithienyl (BBTOAc), and 5-(3,4-diacetoxy-1-butynyl)-2,2′-bithienyl [BBT(OAc)2], were analysed by GC–MS. The proposed SFE method allowed the selective extraction of thiophenes in 60 min dynamic time with supercritical CO2 without modifier co-solvent, at 30 MPa and 40 °C. The SFE and the reference solvent extraction yielded similar results. The SFE of intact roots and flowers yielded 717 ± 31.3 and 480 ± 26.6 μg g−1 α-T, respectively, while the leaves did not contain considerable amounts of thiophenes. Remarkable amounts of BBT, BBTOAc, and BBT(OAc)2 were characteristic of the SFE of hairy root cultures.





Similar content being viewed by others
References
Croes AF, Jacobs JJMR, Arroo RRJ, Wullems GJ (1994) Plant Cell Tissue Organ Cult 38:159–165. doi:10.1007/BF00033873
Arroo RRJ, Jacobs JJMR, de Konig EAH, de Waard M, van de Westerlo E, van Galen PM, Swolfs AEM, Klunder AJH, Croes AF, Wullems GJ (1995) Phytochemistry 38:1193–1197. doi:10.1016/0031-9422(94)00763-J
Margl L, Eisenreich W, Adam P, Bacher A, Zenk MH (2001) Phytochemistry 58:875–881. doi:10.1016/S0031-9422(01)00360-0
Margl L, Tei A, Gyurján I, Wink M (2002) Z Naturforch C 57:63–71
Minto RE, Blacklock BJ (2008) Prog Lipid Res 47:233–306. doi:10.1016/j.plipres.2008.02.002
Hudson JB, Towers GHN (1991) Pharmacol Ther 49:181–222. doi:10.1016/0163-7258(91)90055-Q
Hudson JB, Graham EA, Rossi R, Carpita A, Neri D, Towers GHN (1993) Planta Med 59:447–450. doi:10.1055/s-2006-959729
Romagnoli C, Mares D, Fasulo MP, Bruni A (1994) Phytother Res 8:332–336. doi:10.1002/ptr.2650080604
Mares D, Tosi B, Romagnoli C, Poli F (2002) Pharm Biol 40:400–404. doi:10.1076/phbi.40.5.400.8459
Mares D, Tosi B, Poli F, Andreotti E, Romagnoli C (2004) Microbiol Res 159:295–304. doi:10.1016/j.micres.2004.06.001
Buena AP, Díez-Rojo MÁ, López-Pérez JA, Robertson L, Escuer M, Bello A (2008) Crop Prot 27:96–100. doi:10.1016/j.cropro.2007.04.011
Jin W, Shi Q, Hong C, Cheng Y, Ma Z, Qu H (2008) Phytomedicine 15:768–774. doi:10.1016/j.phymed.2007.10.007
Downum KR, Towers GHN (1983) J Nat Prod 46:98–103. doi:10.1021/np50025a008
Ketel DH (1987) J Exp Bot 38:322–330. doi:10.1093/jxb/38.2.322
Sütfeld R, Towers GHN (1982) Phytochemistry 21:277–279. doi:10.1016/S0031-9422(00)95250-6
Norton RA, Finlayson AJ, Towers GHN (1985) Phytochemistry 24:719–722. doi:10.1016/S0031-9422(00)84883-9
Croes AF, Bosveld M, Wullems GJ (1988) Control of thiophene accumulation in Tagetes. In: Lam J, Breteler H, Arnason T, Hansen L (eds) Chemistry and biology of naturally-occurring acetylenes and related compounds (NOARC). Elsevier, Amsterdam, pp 255–265
Croes AF, van den Berg AJR, Bosveld M, Breteler H, Wullems GJ (1989) Planta 179:43–50. doi:10.1007/BF00395769
Bicchi C, Frattini C, Pellegrino G, Rubiolo P, Raverdino V, Tsoupras G (1992) J Chromatogr A 609:305–313. doi:10.1016/0021-9673(92)80174-S
Lang Q, Wai CM (2001) Talanta 53:771–782. doi:10.1016/S0039-9140(00)00557-9
Zougagh M, Valcárcel M, Ríos A (2004) Trends Analyt Chem 23:399–405. doi:10.1016/S0165-9936(04)00524-2
Herrero M, Cifuentes A, Ibañez E (2006) Food Chem 98:136–148. doi:10.1016/j.foodchem.2005.05.058
Abbas KA, Mohamed A, Abdulamir AS, Abas HA (2008) J Biochem Biotech 4:345–353. doi:10.3844/ajbbsp.2008.345.353
Bicchi C, Binello A, Rubiolo P (1999) Phytochem Anal 10:17–21. doi:10.1002/(SICI)1099-1565(199901/02)10:1<17:AID-PCA429>3.0.CO;2-K
Ma Q, Xu X, Gao Y, Wang Q, Zhao J (2008) Int J Food Sci Technol 43:1763–1769. doi:10.1111/j.1365-2621.2007.01694.x
Gao Y, Nagy B, Liu X, Simándi B, Wang Q (2009) J Supercrit Fluids 49:345–350. doi:10.1016/j.supflu.2009.02.006
Naranjo-Modad S, López-Munguía A, Vilarem G, Gaset A, Bárzana E (2000) J Agric Food Chem 48:5640–5642. doi:10.1021/jf000121i
Wells C, Bertsch W, Perich M (1992) Chromatographia 34:241–248. doi:10.1007/BF02268352
Vasudevan P, Tandon M, Pathak N, Nuennerich P, Mueller F, Mele A, Lentz H (1997) J Sci Ind Res 56:662–672
Szarka SZ, Héthelyi É, Kuzovkina IN, Lemberkovics É, Szőke É (2008) Chromatographia 68:S63–S69. doi:10.1365/s10337-008-0668-5
Murashige T, Skoog F (1962) Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Kuzovkina IN, Mantrova OV, Al’terman IE, Yakimov SA (1996) Russ J Plant Physiol 43:291–298
Héthelyi É, Tétényi P, Kaposi P, Dános B, Kernóczi ZS, Büki GY, Koczka I (1988) Herba Hung 27:89–105
Krishna A, Mallavarapu GR, Kumar S, Ramesh S (2002) J Essent Oil Res 14:433–436
Adams RP (2001) Identification of oil components by gas chromatography/quadrupole mass spectroscopy. Allured Publ. Corp, Carol Stream, IL
Van den Dool H, Kratz PD (1963) J Chromatogr A 11:463–471. doi:10.1016/S0021-9673(01)80947-X
ICH Harmonised Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology Q2(R1) (2005) International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use
Guidance for Industry: Bioanalytical Method Validation (2001) US Department of Health and Human Services, FDA, CDER, CVM
Balla J (2006) A gázkromatográfia analitikai alkalmazásai (Analytical Applications of Gas Chromatography). Edison House, Budapest
Lemberkovics É (2006) Gázkromatográfia alkalmazása (Applications of Gas Chromatography). In: Kalász H, Lengyel J (eds) A gyógyszerek szervezetbeni sorsa és vizsgáló módszerei (The fate of pharmaceutical drugs in living organisms and analytical techniques). Semmelweis Kiadó, Budapest, pp 175–198
Acknowledgments
This work was supported by OMFB-00077/2006 (grant number: RUS-19/04).
Author information
Authors and Affiliations
Corresponding author
Additional information
I. Gyurján: Deceased.
Rights and permissions
About this article
Cite this article
Szarka, S., Gyurján, I., László, M. et al. GC–MS Studies of Thiophenes in the Supercritical Fluid CO2 and Solvent Extracts of Tagetes patula L.. Chroma 71, 1039–1047 (2010). https://doi.org/10.1365/s10337-010-1591-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-010-1591-0