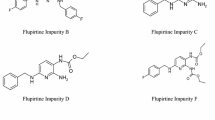
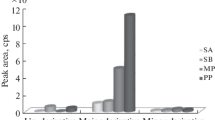
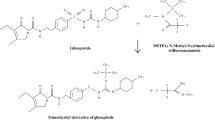
Abstract
A rapid and sensitive gas chromatographic method using flame ionization detection (GC–FID) has been developed and validated for five process related non-chromophoric impurities viz, 2-(2-chloroethoxy)ethanol (2-CEE), piperazine, 2-(piperazin-1-yl)ethanol (HEP), 2-[2-(piperazin-1-yl)ethoxy]ethanol (HEEP), 2,2-[piperazine-1,4-diylbis(ethane-2,1-diyloxy)]diethanol (DEEP) observed during the process development of quetiapine hemifumarate, an antipsychotic drug is presented. All five non-chromophoric impurities ranging from 0.05 to 0.1% were detected using DB-5 (30 m × 0.53 mm, 5 μm) column with a good peak separation. The method was fully validated according to the ICH Q2 (R1) guidelines. The investigated validation protocols showed that the method has acceptable specificity, accuracy, linearity, precision, robustness and high sensitivity with detection limits and quantitation limits ranging from 0.001 to 0.01% and 0.004 to 0.03%, respectively. These non-chromophoric impurities generated during the process were identified by GC–MS and are characterized by MS, 1H NMR and FT-IR spectroscopy.


Similar content being viewed by others
References
(a) Edward JW, Wilmington D (1989) US Pat No. 4879288, (b) http://www.astrazeneca.com/_mshost3690701/content/resources/media/investors/az-june-2008-goldmansachs.pdf, (c) http://www.news-medical.net/news/20091106/Bipolar-disorder-drug-market-to-decline-from-2463-billion-to-245-billion.aspx
Campbell A, Yeghiayana S, Baladessarini RJ, Neumeyer JL (1991) Psychopharmacology 103:323–329
Saller CF, Salama AI (1993) Psychopharmacology 112:285–292
Goldstein JM, Litwin LC, Sutton EB, Malick JB (1993) Psychopharmacology 112:293–298
Migler BM, Warawa EJ, Malick JB (1993) Psychopharmacology 112:299–307
Copur M, Arpaci B, Demir T, Narin H (2007) Clin Drug Investig 27(2):30–123
Shaw JA, Lewis JE, Pascal S, Sharma RK, Rodriguez RA, Guillen R, Pupo-Guillen M (2001) J Child Adolesc Psychopharmacol 11:415–424
Pullen RH, Palermo KM, Curtis MA (1992) J Chromatogr 573:49–57
Kumar KV, Kanhaiya L, Suhail A, David L (2006) WO Pat appl No. 113425
Fathalla Belal, Elbrashy A, Manal Eid, Nasar JJ (2008) J Liq Chromatogr Related Technol 31:1283–1298
Sachse J, Koller J, Hartter S, Hiemke C (2006) J Chromatogr B 830:342–348
Bharathi CH, Prabahar KJ, Prasad ChS, Rao MS, Trinadhachary GN, Handa VK, Dandala R, Naidu A (2008) Pharmazie 63:14–19
Stolarczyk EU, Kaczmarek L, Eksanow K, Kubiszewski M, Glice M, Kutner A (2009) Pharm Dev Techonol 14(1):27–37
Krishna SR, Rao BM, Rao NS (2008) Rasayan J Chem 1(3):466–474
Raju IV S, Raghuram P, Sriramulu (2009) Chromatographia 70:545–550
Stolarczyk EU, Groman A, Kaczmarek LS, Golebiewski P (2007) Acta Pol Pharm 64:187–189
Niphade NC, Mali AC, Pandit BK, Jagtap KM, Jadhav SA, Jachak MN, Mathad VT (2009) Org Process Res Dev 13(4):792–797
Validation of Compendial Methods. The United States Pharmacopeia (2008) 31st edn. USP 31, Section <1225>
ICH, Validation of Analytical Procedures; Text and Methodology, Q2 (R1).
Acknowledgments
The authors wish to thank the management of the Megafine group for supporting this work. We would also like to thank colleagues in the Research and Development division of Megafine Pharma (P) for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jadhav, S.A., Landge, S.B., Shelke, S.S. et al. Original GC–FID Determination of Non-Chromophoric Impurities in Quetiapine: An Antipsychotic Drug. Chroma 71, 1055–1061 (2010). https://doi.org/10.1365/s10337-010-1585-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-010-1585-y