Abstract
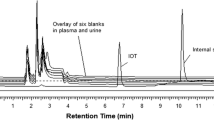
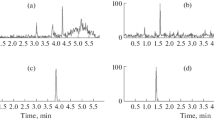
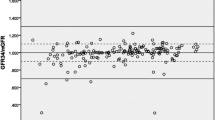
A rapid high-performance liquid chromatography UV method and a simple sample preparation for analyzing iohexol in canine plasma, for evaluating glomerular filtration rate (GFR) and intestinal permeability, were developed and validated. Trifluoroacetic acid (TFA) was used for protein precipitation and iohexol extraction from plasma, followed by vortex mixing and centrifugation. As an internal standard, 4-aminobenzoic acid (para-aminobenzoic acid, PABA) was added. The supernatant (5 μL) was injected into a Zorbax SB-C18 LC column maintained at 50 °C. The mobile phase of the LC method was a water–methanol gradient at pH 3.0 adjusted with TFA. Fast LC measurement was achieved by using a rapid-resolution LC technique. Total run time was 13 min, and UV wavelength was set at 246 nm. Precision of the method was 0.2–9.0%, depending on the iohexol concentration in plasma. Recovery of iohexol from plasma was over 90%, and recovery of the internal standard 99.1 ± 1.4%. The calibration curve was linear (r = 0.9997) over iohexol concentrations of 2.5–150 μg mL−1 (n = 5). This method is fast, simple, reliable and applicable in clinical settings.

Similar content being viewed by others
References
Hecht S, Daniel GB, Mitchell SK (2006) Vet Radiol Ultrasound 47:602–608. doi:10.1111/j.1740-8261.2006.00194.x
Kunze C, Bahr A, Lees GE (2006) Vet Radiol Ultrasound 47:103–107. doi:10.1111/j.1740-8261.2005.00115.x
Sandersson LS (2009) Measuring glomerular filtration rate: practical use of clearance tests. In: Bonagura JD (ed) Kirk′s current veterinary therapy XIV. Saunders Elsevier, Ohio, pp 869–871
Schwartz GJ, Furth SL (2007) Pediatr Nephrol 22:1839–1848. doi:10.1007/s00467-006-0358-1
Goy-Thollot I, Chafotte C, Besse S, Garnier F, Barthez PY (2006) Vet Radiol Ultrasound 47:168–173. doi:10.1111/j.1740-8261.2006.00133.x
Bexfield NH, Heiene R, Gerritsen RJ, Risøen U, Eliassen KA, Herrtage ME, Michell AR (2008) J Vet Intern Med 22:66–73. doi:10.1111/j.1939-1676.2007.0035.x
Halme L, Turunen U, Tuominen J, Forsstrom T, Turpeinen U (2000) Scand J Clin Lab Invest 60:695–701. doi:10.1080/00365510050216420
Halme L, Edgren J, Turpeinen U, von Smitten K, Stenman UH (1997) Scand J Gastroenterol 32:148–152. doi:10.3109/00365529709000185
Klenner S, Coenen M, Failing K, Hewicker-Trautwein M, Ternes W, Verspohl J, Spillmann T (2009) Vet Clin Pathol 38:353–360. doi:10.1111/j.1939-165X.2009.00136.x
Andersen R, Stordahl A, Aase S, Laerum F (2001) Dig Dis Sci 46:208–213. doi:10.1023/A:1005630429723
Frias R, Ouwehand A, Spillmann T, Vankerckhovend V, Hewicker-Trautwein M, Salminen S, Gueimonde M (2009) Food Res Int 42:636–640. doi:10.1016/j.foodres.2009.02.004
O’Dell-Anderson KJ, Twardock R, Grimm JB, Grimm KA, Constable PD (2006) Vet Radiol Ultrasound 47:127–135. doi:10.1111/j.1740-8261.2006.00118.x
Klenner S, Bergmann C, Strube K, Ternes W, Spillmann T (2007) Chromatographia 65:733–736. doi:10.1365/s10337-007-0202-1
Frenneby B, Sterner G (2002) Eur Radiol 12:475–484. doi:10.1007/s003300100864
Nilsson-Ehle P (2001) eJIFCC (online computer file) 13(2) International Federation of Clinical Chemistry and Laboratory Medicine. http://www.ifcc.org/index.asp?cat=Publications&scat=eJIFCC_&suba=Vol_13_No_2&subx=Iohexol_clearance_for_the_determination_of_glomerular_filtration_rate-_15_years_experience_in_clinical_practice_&zip=1&dove=1&zona=full&numero=&aq=1
Laroute V, Lefebvre HP, Costes G, Toutain PL (1999) J Pharmacol Toxicol Methods 41:17–25. doi:10.1016/S1056-8719(99)00016-7
Soman RS, Zahir H, Akhlaghi F (2005) J Chromatogr B 816:339–343. doi:10.1016/j.jchromb.2004.11.046
Meucci V, Gasperini A, Soldani G, Guidi G, Giorgi M (2004) J Chromatogr Sci 42:107–111
Farthing D, Sica DA, Fakhry I, Larus T, Ghosh S, Farthing C, Vranian M, Gehr T (2005) J Chromatogr B 826:267–272. doi:10.1016/j.jchromb.2005.05.050
ICH Guidelines (2005) Q2 (R1): validation of analytical procedures: text and methodology. http://www.ich.org/
Huber L (2007) Validation and qualification in analytical laboratories. Informa Healthcare, New York, pp 125–154
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pöytäkangas, M., Saario-Paunio, E., Putkonen, T. et al. Rapid LC-UV Analysis of Iohexol in Canine Plasma for Glomerular Filtration Rate Determination. Chroma 71, 211–216 (2010). https://doi.org/10.1365/s10337-009-1445-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-009-1445-9