Abstract
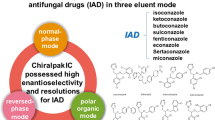
The solvent versatility of Chiralpak IA (amylose tris(3,5-dimethylphenylcarbamate)), Chiralpak IB (cellulose tris(3,5-dimethylphenylcarbamate)) and Chiralpak IC (cellulose tris(3,5-dichlorophenylcarbamate)) immobilized onto silica gel, are investigated for the enantioselective separation of a set of acidic drugs in liquid chromatography. Non-standard LC organic solvents like dichloromethane, ethyl acetate, tetrahydrofuran, methyl-tert-butyl ether were used in mobile phase compositions and/or diluent agent for the analyte on all new columns. Furthermore, the enantioselective separations of the reported compounds were compared on both immobilized columns (Chiralpak IA and IB) and their conventionally coated versions (Chiralpak AD-H and Chiralcel OD-H, respectively), using a mixture of n-hexane/2-PrOH/TFA (80:20:0.1 v/v/v). The versatility of the immobilized Chiralpak IB in monitoring reactions performed in non-standard solvent was studied on a representative example consisting of the lipase-catalyzed enantioselective esterification of flurbiprofen with n-butanol in methyl-tert-butyl ether as organic solvent.





Similar content being viewed by others
References
Lane RM, Baker GB (1999) Cell Mol Neurobiol 19:355–372. doi:10.1023/A:1006997731966
Rouhi AM (2004) Chem Eng News 82:47–62. http://pubs.acs.org/cen/coverstory/8224/8224chiral.html
Ghanem A, Naim L (2006) J Chromatogr A 1101:171–178. doi:10.1016/j.chroma.2005.09.081
Ghanem A, Ginatta C, Jiang Z, Schurig V (2003) Chromatographia 57:275–281. doi:10.1007/BF02492116
Ghanem A, Schurig V (2001) Chirality 13:118–123. doi:10.1002/1520-636X(2001)13:2<118::AID-CHIR1007>3.0.CO;2-Q
Cirilli R, Ferretti R, Gallinella B, La Torre F, La Regina G, Silvestri R (2005) J Sep Sci 28:627–634. doi:10.1002/jssc.200400102
Zhang Y, Wu DR, Wang-Iverson DB, Tymiak AA (2005) Drug Discov Today 10:571–577. doi:10.1016/S1359-6446(05)03407-0
Berthod A (2006) Anal Chem 1:2093–2099. doi:10.1021/ac0693823
Ghanem A, Lacrampe F, Schurig V (2005) Helv Chim Act 88:216–239. doi:10.1002/hlca.200590003
Wainer IW, Stiffin RM, Shibata T (1987) J Chromatogr 411:139–151. doi:10.1016/S0021-9673(00)93965-7
Aboul-Enein HY, Ali I (2003) Chiral separation by liquid chromatography and related technologies. Marcel Dekker, NewYork, pp 338–358
Spitzer T, Yashima E, Okamoto Y (1999) Chirality 11:195–200. http://www3.interscience.wiley.com/journal/40002935/abstract
Wainer IW, Alembik MC (1986) J Chromatogr 358:85–93. doi:10.1016/S0021-9673(01)90318-8
Okamoto M, Nakazawa H (1991) J Chromatogr 588:177–180. doi:10.1016/0021-9673(91)85020-G
Balmer V, Lagerstrom PO, Persson BA, Schill V (1992) J Chromatogr 592:331–337. doi:10.1016/0021-9673(92)85104-2
O’Brien T, Crocker L, Thompson R, Thompson K, Toma PH, Conlon DA, Feibush B, Moeder C, Bicker G, Grinberg N (1997) Anal Chem 69:1999–2007. doi:10.1021/ac961241l
Krstulovic AM, Fouchet MH, Burke JT, Gillet G, Durand A (1988) J Chromatogr 452:477–483. doi:10.1016/S0021-9673(01)81470-9
McCarthy JP (1994) J Chromatogr A 685:349–355. doi:10.1016/0021-9673(94)00803-5
Okamoto Y, Kawashima M, Aburatani R, Hatada K, Nishiyama T, Masuda M (1986) Chem Lett 15:1237–1240. doi:10.1246/cl.1986.1237
Okamoto Y, Aburatani R, Kaida Y, Hatada K (1988) Chem Lett 17:1125–1128. doi:10.1246/cl.1988.1125
Okamoto Y, Yashima E (1998) Angew Chem Int Ed Engl 37:1020–1043. doi:10.1002/(SICI)1521-3773(19980504)37:8<1020::AID-ANIE1020>3.0.CO;2-5
Chankvetadze B, Yamamoto C, Okamoto Y (2001) J Chromatogr A 922:127–137. doi:10.1016/S0021-9673(01)00958-X
Yashima V, Fukaya H, Okamoto Y (1994) J Chromatogr A 677:11–19. doi:10.1016/0021-9673(94)80539-3
Franco P, Senso A, Oliveros L, Minguillon C (2001) J Chromatogr A 906:155–170. doi:10.1016/S0021-9673(00)00531-8
Ghanem A (2006) J Chromatogr A 1132:329–332. doi:10.1016/j.chroma.2006.09.043
Zhang T, Schaeffer M, Franco P (2005) J Chromatogr A 1083:96–101. doi:10.1016/j.chroma.2005.06.003
Francotte ER (2001) J Chromatogr A 906:379–397. doi:10.1016/S0021-9673(00)00951-1
Chankvetadze B, Ikai T, Yamamoto C, Okamoto Y (2004) J Chromatogr A 1042:55–60. doi:10.1016/j.chroma.2004.05.011
Chen X, Liu Y, Qin F, Kong L, Zou H (2003) J Chromatogr A 1010:185–194. doi:10.1016/S0021-9673(03)01104-X
Cirilli R, Simoneli A, Ferretti R, Bolasco A, Chimenti P, Secci D, Maccioni E, La Torre F (2006) J Chromatogr A 1101:198–203. doi:10.1016/j.chroma.2005.10.003
Zhang T, Nguyen D, Franco P, Murakami T, Ohnishi A, Kurosawa H (2006) Anal Chim Acta 557:221–228. doi:10.1016/j.aca.2005.10.017
Hayball PJ (1996) Drugs 52:47–58. http://ejournals.ebsco.com/Journal2.asp?JournalID=101134
Alcantara AR, Sanchez-Montero JM, Sinisterra JV (2000) Chemoenzymatic preparation of enantiomerically pure S(+)-2-arylpropionic acids. In: Patel RN (ed) Stereoselective biocatalysis. Marcel Dekker, New York, pp 659–702
Bhandarkar SV, Neau SH (2000) Electron J Biotechnol 3:195–201. Available from: http://www.ejbiotechnology.info/content/vol3/issue3/full/3/index.html
Brune K, Beck WS, Geisslinger G, Menzel-soglowek S, Peskar BM, Peskar BA (1991) Experientia 47:257–261
Geisslinger G, Ferreira SH, Menzel S, Schlott D, Brune K (1994) Life Sci 54:173–177. doi:10.1016/0024-3205(94)00555-9
Wechter WJ (1994) J Clin Pharmacol 34:1036–1042
Klibanov AM (1989) Trends Biochem Sci 14:141–144. doi:10.1016/0968-0004(89)90146-1
Dordick JS (1989) Enzyme-Microb Technol 14:194–211. doi:10.1016/0141-0229(89)90094-X
Duan G, Ching CB (1998) Biochem Eng J 2:237–245. doi:10.1016/S1369-703X(98)00037-0
Arroyo M, Sinisterra JV (1994) J Org Chem 59:4410–4417. doi:10.1021/jo00095a014
Morrone R, Nicolosi G, Patti A, Piattelli M (1995) Tetrahedron Asymmetry 6:1773–1778. doi:10.1016/0957-4166(95)00223-C
Ghanem A, Aboul-Enein MN, El-Azzouny A, El-Behairy MF, Al-Humaidi E, Al-Ahdal MN, Alaidan AA, Amin K (2008) Chirality 20:871–877. doi:10.1002/chir.20533
Ghanem A, Aboul-Enein HY (2005) J Liquid Chromatogr Relat Tech 28:2863–2874. doi:10.1080/10826070500269919
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Paul Haddad on the Occasion of his 60th Birthday.
Rights and permissions
About this article
Cite this article
Ghanem, A., Aboul-Enein, M.N., El-Azzouny, A. et al. Solvent Versatility of Immobilized Amylose and Cellulose-Based Chiral Stationary Phases in Enantioselective LC Separation and Monitoring of Bio-Catalyzed Resolutions of Acidic Drugs in Non-Standard Organic Solvents. Chroma 70, 349–363 (2009). https://doi.org/10.1365/s10337-009-1201-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-009-1201-1