Abstract
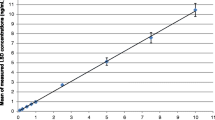
A rapid and sensitive liquid chromatography-tandem mass spectrometry assay was developed for the determination of a novel histone deacetylase inhibitor, cyclo{(2S)-2-amino-8-[(aminocarbonyl)hydrazono]decanoyl-1-l-tryptophyl-l-isoleucyl-(2R)-2-piperidinecarbonyl} (SD-2007), in rat serum. The mobile phase consisted of acetonitrile and ammonium formate (10 mM) (85:15 v/v), and the flow rate was 0.25 mL min−1. Chromatographic separations were achieved by isocratic elution on a C18 column. Multiple reaction monitoring was based on the transition of m/z = 681.8 → 83.6 for SD-2007 and 372.1 → 176.1 for trazodone (internal standard). A linearity was observed over a concentration range from 2 to 1,000 ng mL−1 (r 2 > 0.999), with the lower limit of quantification at 2 ng mL−1 with 100 μL of rat serum. The mean intra- and inter-day assay accuracy ranged from 98.5–109.7% to 95.2–102.7%, respectively, and the mean intra- and inter-day precision was between 4.3–11.3% and 2.9–13.3%. The developed assay was applied to a pharmacokinetic study of SD-2007 in rats after intravenous injection (dose 4 mg kg−1).


Similar content being viewed by others
References
Drummond DC, Noble CO, Kirpotin DB, Guo Z, Scott GK, Benz CC (2005) Annu Rev Pharmacol Toxicol 45:495–528
Dokmanovic M, Marks PA (2005) J Cell Biochem 96:293–306
Marks PA, Richon VM, Rifkind RA (2000) J Natl Cancer Inst 92:1210–1216
Krämer OH, Göttlicher M, Heinzel T (2001) Trends Endocrinol Metab 12:294–300
Kouraklis G, Theocharis S (2002) Curr Med Chem Anticancer Agents 2:477–484
Vigushin DM, Coombes RC (2004) Curr Cancer Drug Targets 4:205–218
Mei S, Ho AD, Mahlknecht U (2004) Int J Oncol 25:1509–1519
Rasheed WK, Johnstone RW, Prince HM (2007) Expert Opin Investig Drugs 16:659–678
Mai A, Massa S, Rotill D, Cerbara I, Valente S, Pezzi R, Simeoni S, Ragno R (2005) Med Res Rev 25:261–309
Han JW, Ahn SH, Park SH, Wang SY, Bae GU, Seo DW, Kwon HK, Hong S, Lee HY, Lee YW, Lee HW (2000) Cancer Res 60:6068–6074
Ueda T, Takai N, Nishida M, Nasu K, Narahara H (2007) Int J Mol Med 19:301–308
Lee HY, Jung YH, Han JW, Lee SY, Lee YW, Lee HY, Zee OP, PCT No. WO 02/051846 (2002)
Yoo EJ, Lee BM (2007) J Toxicol Environ Health Part A 68:2079–2109
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Anal Chem 75:3019–3030
Mei H, Hsieh Y, Nardo C, Xu X, Wang S, Ng K, Korfmacher WA (2003) Rapid Commun Mass Spectrom 17:97–103
Mallet CR, Lu Z, Mazzeo JR (2004) Rapid Commun Mass Spectrom 18:49–58
Shin BS, Kim J, Yoon CH, Kim CH, Park EH, Han JW, Yoo SD (2005) Rapid Commun Mass Spectrom 19:408–414
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shin, B.S., Hong, D.K., Kwak, J.H. et al. LC-MS-MS Determination of Cyclo{(2S)-2-amino-8-[(aminocarbonyl)hydrazono] decanoyl-1-l-tryptophyl-l-isoleucyl-(2R)-2-piperidinecarbonyl} a Novel Histone Deacetylase Inhibitor in Rat Serum. Chroma 67, 231–235 (2008). https://doi.org/10.1365/s10337-007-0495-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-007-0495-0