Abstract
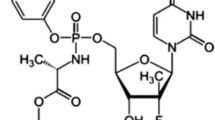
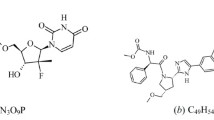
This study describes the development and full validation of a stability-indicating HPLC method to quantify ritonavir (RTV) and lopinavir (LPV) in soft gelatin capsules. The method uses a LiChrospher® 100 RP-18 (250 mm × 4.6 mm, 5 µm, Merck) column and isocratic elution. The mobile phase consisted of a mixture of acetonitrile-water-methanol (53:37:10, v/v/v), pumped at a flow-rate of 1.0 mL min−1 and UV detection at 210 nm using a photodiode array detector. LPV and RTV were exposed to thermal, photolytic, hydrolytic and oxidative stress conditions, and the stressed samples were analyzed by the proposed method. The response was linear over a range of 40-360 µg mL−1 for LPV and 10–90 µg mL−1 for RTV (r > 0,999 for both drugs). The mean recoveries were 99.46 and 100.81% for LPV and RTV, respectively. The RSD values for intra- and inter-day precision studies were < 0.70% for both drugs. Degradation studies showed that lopinavir is stable in thermal, alkaline and oxidative conditions, while ritonavir degraded under these conditions. The method was found to be stability-indicating and can be used for the routine analysis of the association LPV/RTV in soft gelatin capsules.
Similar content being viewed by others
References
Sethi ML (2002) Antiviral Agents and Protease Inhibitors. In: Williams DA, Lemke TL Foye's Principles of Medicinal Chemistry, Lippincott Williams & Wilkins, Philadelphia, pp. 967–975
Raffanti S, Hass DW (2003) Antimicrobial Agents-Antiretroviral Agents. In: Hardman JG, Limbird LE (eds) Goodman & Gilman's: The Pharmacological Basis of Therapeutics, McGraw-Hill, Brasil, pp. 1212–1033
França FFAC (2004) Andrejus Korolkovas: Dicionário Terapêutico Guanabara, Guanabara Koogan, Brasil, pp. 18.36–18.54
Abbott Laboratories, Kaletra® Product Monograph http:// www.rxabbott.com/PDF/kaletrapi.pdf
US Department of Health and Human Services Guidelines for use of antiretroviral agents in HIV-1 infected adults and adolescents. http:// www.aidsinfo.nih.gov
Sham HL, Kempf DJ, Molla A, Marsh KC, Kumar GN, Chen CM, Kati W, Stewart K, Lal R, Hsu A, Betebenner DA, Korneyeva M, Vasavanonda S, McDonald E, Saldivar A, Wideburg N, Chen X, Niur P, Park O, Jayanti V, Grabowksi B, Granneman GR, Sun E, Japour AJ Leonard JM, Plattner, JJ, Norbeck, DW (1998) Antimicrob Agents Chemother 42:3218–24
Molla A, Vasavanonda S, Kumar GN, Sham HL, Johnson M, Grabowski B, Denissen JF, Kohlbrenner W, Platnner JJ, Leonard JM, Norbeck DW, Kempf DJ (1998) Virology 63:250–255
Cvetkovic RS and Goa KL (2003 Drugs 63:769–802
Ray J, Pang E, Carey D (2002) J Chromatogr B Biomed Sci Appl 775: 225–230
Justesen US, Pedersen C, Klitgaard, NA (2002) J Chromatogr B Biomed Sci Appl 783:491–500
Titier K, Lagrange F, Pehourcq F, Edno-Mcheik L, Moore N, Molimard M (2002) Ther Drug Monit 24: 417–424
Faux J, Venisse N, Moal G, Dupuis A, Bouquet S (2003) Chromatographia 59:421–426
Usami Y, Oki T, Nakai M, Sagisaka M, Kaneda T (2003) Chem Pharm Bull 51:715–718
Dailly E, Raffib F, Jollieta P (2004) J Chromatogr B Biomed Sci Appl 813: 353–358
Naser L, Rezk NL, Tidwell RR, Kashuba ADM (2004) J Chromatogr B Biomed Sci Appl 805: 241–247
Takahashi M, Yoshida M, Oki T, Okumura N, Suzuki T, Kaneda, S (2005) Biol Pharm Bull 28:1286–1290
Dias LC, Rossi, RC, Donato EM, Bergold AM, Fröehlich. PE (2005) Chromatographia 62: 589–593
ICH (2003) International Conference on Harmonization, Guideline on Stability Testing of New Drug Substances and Products (Q1A)
ICH (1994) International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, Text on Validation of Analytical Procedures (Q2A)
ICH (1996) International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, Guideline on Validation of Analytical Procedures: Methodology, (Q2B)
FDA (1994) Center for Drug Evaluation and Research. Reviewer Guindance: Validation of Chromatographic Methods, Rockville, MD
USP (2005) The United States Pharmacopoeia, United States Pharmacopoeial Convention,Rockville, MD
Watson DG Pharmaceutical Analysis. A Textbook for Pharmacy Students and Pharmaceutical Chemists. Edinburgh: Elsevier Churchill Livingstone, 2005.
Snyder LG, Kirkland JJ, Glajch JL (1997). Practical HPLC method development, John Willey & Sons, USA, pp. 21–57.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Donato, E.M., Dias, C.L., Rossi, R.C. et al. LC Method for Studies on the Stability of Lopinavir and Ritonavir in Soft Gelatin Capsules. Chroma 63, 437–443 (2006). https://doi.org/10.1365/s10337-006-0785-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-006-0785-y