Abstract
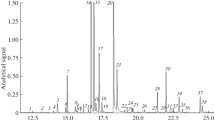
The production and the composition of the compounds (mono-, sesquiterpenes and acetylenic thiophenes) obtained by the steam distillation of Tagetes patula L. have been investigated. The volatile oil was produced by steam distillation. GC was carried out on three types of stationary phase using flame ionization and mass selective detection. Percentage data were calculated by the area normalization method with very good repeatability (RSD below 5%). Oils from flower-heads were rich in β-caryophyllene (53.5%) and the leaves contained terpinolene in high concentration (21.1%). The main volatile component of the hairy roots and intact roots was 5-(3-buten-1-ynyl)-2,2'-bithienyl (BBT) yielding 28.5% and 44.0% in the oils. Three new minor constituents were identified as α-gurjunene, β-caryophyllene and (E)-β-farnesene. A flash chromatographic method was developed for the isolation of thiophenes from a solvent extract of intact roots. The collected fractions were screened by TLC and analyzed by GC-MS. Three thiophene fractions were obtained containing BBT, 5-(4-acetoxy-1-butynyl)-2,2'-bithienyl (BBTOAc) and 5-(4-hydroxy-1-butynyl)-2,2'-bithienyl (BBTOH).
Similar content being viewed by others
References
Piccaglia R, Marotti M, Grandi S (1998) Ind Crop Prod 8:45–51
Mukundan U, Hjortso M (2001) Biotechnol Agric For 48:274 293
Hudson JB, Graham EA, Rossi R, Carpita A, Neri D, Towers GHN (1993) Planta Med 59:447–450
Hudson JB, Towers GHN (1991) Pharmac Ther 49:181–222
Mares D, Tosi B, Poli F, Andreotti E, Romagnoli C (2004) Microbiol Res 159:295–304
Suresh B, Rajasekaran T, Ramachandra Rao S, Raghavarao KSMS, Ravishankar GA (2001) Process Biochemistry 36:987–993
Croes AF, Jacobs JMRJ, Arroo RRJ, Wullems J (1994) Plant Cell Tissue and Organ Cult 38:159–165
Talou JR, Cascone O, Giulietti AM (1994) Planta Med 60:260–262
Norton RA, Finlayson AJ, Towers GHN (1985) Phytochemistry 24:719–722
Gamborg OK, Miller RA, Ojima K (1968) Exptl Cell Res 50:151–158
In: (2004) European Pharmacopoeia 5th. Edition Vol. 2. EDQM, Strasbourg, France pp. 1977–1978
Bicchi C, Frattini C, Pellegrino G, Rubiolo P, Raverdino V, Tsoupras G (1992) J Chromatogr 609:305–313
Margl L, Tei A, Gyurján I, Wink M (2002) Z Naturforsch C 57:63–71
Krishna A, Mallavarapu GR, Kumar S, Ramesh S (2002) J Essent Oil Res 14:433–436
Adams RP (2001) In: Identification of Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Allured Publ. Corp., Carol Stream, IL
Héthelyi É, Tétényi P, Kaposi P, Dános B, Kernóczi ZS, Büki GY (1988) Herba Hung 2–3:89–105
Margl L, Eisenreich W, Adam P, Bacher A, Zenk MH (2001) Phytochemistry 58:875–881
Balla J (1997) In: A gázkromatográfia analitikai alkalmazásai Abigél Bt, Budapest, Hungary
Szarka Sz, Héthelyi É, Lemberkovics É, Bálványos I, Szõke É. J Essent Oil Res (submitted for publication)
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at: 6th Balaton Symposium on High-Performance Separation Methods, Siófok, Hungary, September 7–9, 2005
Rights and permissions
About this article
Cite this article
Szarka, S., Héthelyi, É., Lemberkovics, É. et al. GC and GC-MS Studies on the Essential Oil and Thiophenes from Tagetes patula L.. Chroma 63 (Suppl 13), S67–S73 (2006). https://doi.org/10.1365/s10337-006-0729-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-006-0729-6