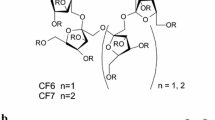
Abstract
Direct reversed-phase high-performance liquid chromatographic methods were developed for the separation of enantiomers of β-lactams. The enantiomers of 7 aryl-substituted β-lactams were separated on chiral stationary phases containing the macrocyclic glycopeptide antibiotic teicoplanin (Chirobiotic T) and teicoplanin aglycone (Chirobiotic TAG) at 10-°C increments in the range 5–45 °C, using different compositions of 0.1% aqueous triethylammonium acetate (pH 4.1)/methanol (v/v) as mobile phase. The mobile phase composition and temperature were varied to achieve baseline resolutions in a single chromatographic run. The dependence of the natural logarithms of the selectivity factors ln α on the inverse of temperature, 1/T, was used to determine the thermodynamic data on the enantiomers. The thermodynamic data revealed that all the compounds in this study undergo separation via the same enthalpy-driven chiral recognition mechanism. The different methods were compared in systematic chromatographic examinations. The effects of the organic modifier, the mobile phase composition and the temperature on the separation were investigated.
Similar content being viewed by others
References
Achilles K, Schirmeister T, HH Otto (2000) Arch Pharm Med Chem 333:243–253
Lee HK, Chun JS, Pak CS (2001) Tetrahedron Lett 42:3483–3486
Sonnet P, Dallemagne P, Guillon J, Enguehard C, Stiebing S, Tanguy J, Bureau R, Rault S, Auvray P, Moslemi S, Sourdaine P, Séralini GE (2000) Bioorg Med Chem 8:945–955
Palomo C, Aizpurua JM, Ganboa I (1997) In: Enantioselective Synthesis of beta-Amino Acids, Juaristi E (ed) Wiley-VHC, New York, pp 279
Fülöp F (2001) Chem Rev 101:2181–2204
Fülöp F, Forró E, Tóth GK (2004) Org Lett 6:4239–4248
Pirkle WH, Finn JM, Schreiner JL, Hamper BC (1981) J Amer Chem Soc 103:3964–3966
Pirkle WH, Tsipouras A, Huyn MH, Hart DJ, Lee CS (1986) J Chromatogr 358:377–384
Pirkle WH, Spence PL (1998) Chirality 10:430–433
Lee CS, Chen HH (1994) J Chinese Chem Soc 41:187–190
Okamoto Y, Hatada K (1990) Jpn Kokai Tokkyo Koho 1–9
Okamato Y, Senoh T, Nakane H, Hatada K (1989) Chirality 1:216–222
Okamato Y, Kaida Y (1994) J Chromatogr A 666:403–419
Ficarra R, Calabro ML, Alcaro S, Tomassini S, Melardi S, Ficarra P (2000) Chromatographia 51:411–416
Cirilli R, Del Guidice MR, Ferretti R, La Torre F (2001) J Chromatogr A 923:27–36
Péter A, Árki A, Forró E, Fülöp F, Armstrong DW (2005) Chirality 17:193–200
Huang T, Kuang C, Zhou J, Gou D (1991) Fenxi Huaxue 19:687–689
Péter A, Török G, Fülöp F (1998) J Chromatogr Sci 36:311–317
Yashima E, Shavattanapong P, Okamoto Y (1996) Chirality 8:446–451
Fornstedt T, Sajonz P, Guichon G (1997) J Am Chem Soc 119:1254–1264
Pirkle WH, Burke JA (1991) J Chromatogr A 557:173–185
Péter A, Török G, Armstrong DW, Tóth G, Tourwé D (1998) J Chromatogr A 828:177–190
Péter A, Vékes E, Armstrong DW (2002) J Chromatogr A 958:89–107
Palomo C, Oiarbide M, Bindi S (1998) J Org Chem 63:2469–2474
Forró E, Fülöp F (2003) Org Lett 5:1209–1212
Árki A, Tourwé D, Solymár M, Fülöp F, Armstrong DW, Péter A (2004) Chromatographia 60:43–54
Berthod A, Chen X, Kullman JP, Armstrong DW, Gasparrini F, D'Aquarica I, Villani C, Carotti A (2000) Anal Chem 72:1767–1780
Péter A, Árki A, Tourwé D, Forró E, Fülöp F, Armstrong DW (2004) J Chromatogr A 1031:159–170
Péter A, Török R, Armstrong DW (2004) J Chromatogr A 1057:229–235
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at: 6th Balaton Symposium on High-Performance Separation Methods, Siófok, Hungary, September 7–9, 2005.
Rights and permissions
About this article
Cite this article
Berkecz, R., Ilisz, I., Forró, E. et al. LC Enantioseparation of Aryl-Substituted β-Lactams Using Variable-Temperature Conditions. Chroma 63 (Suppl 13), S29–S35 (2006). https://doi.org/10.1365/s10337-005-0700-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-005-0700-y