Abstract
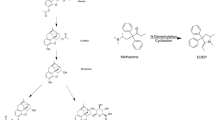
Chiral capillary electrophoresis has been validated for the quantitative analysis of R-(-)-deprenyl (selegiline) and seven of its metabolites, among them the diastereomeric pair of selegiline-N-oxide in rat urine. Linear calibration curves were obtained over the concentration range: 0.5–100 μM for selegiline, N-desmethylselegiline, methamphetamine, amphetamine, and selegiline-N-oxides, and from 0.1-100 μM for para-hydroxylated derivatives of N-desmethylselegiline, methamphetamine, and amphetamine. The inter and intra-assay precision and accuracy varied by <15% for all analytes at concentrations of 2.5, 10 and 25 μM, and <20% at the lower limit of quantification (0.5 or 0.1 μM). The sample extraction procedure was optimised, and sample recoveries ranged: 80–111% and 74–91% at concentrations of 1 and 10 μM, respectively. The extracted urine samples retained quantitative accuracy for at least 5 days after storage at 4 °C. The validated method was used for in vivo metabolism studies in rats treated with either single or repeated dose of selegiline, or selegiline-N-oxide. Stereoselective N-oxidation of selegiline and rapid urinary excretion of selegiline-N-oxides have been observed. The most abundant metabolites of selegiline were the desalkylated and para-hydoxylated derivatives excreted in both conjugated and unconjugated forms in rat urine.
Similar content being viewed by others
References
Magyar K, Vizi ES, Ecseri Z, Knoll J (1967) Acta Physiol Hung 32:377–387
Magyar K, Haberle D (1999) Neurobiology 7:175–190
Ebadi M, Sharma S, Shavali S, Refaey H (2002) J Neurosci Res 67:285–289
Tatton WG, Chalmers-Redman RM, Tatton N (2003) J Neural Transm 110:509–515
Tatton WG, Chalmers-Redman RM (1996) Neurology 47S3:171–183
Reynolds GP, Elsworth JD, Blau K, Sandler M, Lees AJ, Stern GM (1978) Br J Clin Pharmacol 6:542–544
Heinonen EH, Myllyla V, Sotaniemi K, Lammintausta R, Salonen JS, Anttila M, Savijarvi M, Kottila M, Rinne UK (1989) Acta Neurol Scand 126:93–99
Shin H-S (1997) Drug Metab Disp 25:657–662
Magyar K, Tóthfalusi L (1984) Pol J Pharmacol Pharm 36:373–384
Yoshida T, Yamada Y, Yamamoto T, Kuroiwa Y (1986) Xenobiotica 16:129–136
Szoko É, Magyar K (1996) Int’l J Pharm Adv 1:320–327
Lengyel J, Magyar K, Hollósi I, Bartók T, Báthori M, Kalász H, Fürst Z (1997) J Chromatogr A 762:321–326
Kalász H, Kerecsen L, Knoll J, Pucsok J (1990) J Chromatogr, 499:589–599
Wu RF, Ichikawa Y (1995) FEBS Lett 358:145–148
Katagi M, Tatsuno M, Miki A, Nishikawa M, Nakajima K, Tsuchihashi H (2001) J Chromatogr B 759:125–133
Katagi M, Tatsuno M, Tatsuno H, Miki A, Kamata T, Nishioka H, Nakajima K, Nishikawa M, Tsuchihashi H (2002) Xenobiotica 9:823–831
Tábi T, Magyar K, Szöko É (2003) Electrophoresis 24:2665–2673
Szebeni G, Lengyel J, Szekacs G, Magyar K, Gaal J, Szatmári I (1995) Acta Physiol Hung 83:135–141
Haberle D, Kalász H, Hollósi I, Pucsok J, Csermely T, Magyar K, Tóth-Molnár E, Chromatographia (1999) 50:415–422
Kikura R, Nakahara Y, Kojima S (2000) J Chromatogr B 741:163–173
Slawson MH, Taccogno JL, Foltz RL, Moody DE (2002) J Anal Toxicol 26:430–437
Kim E-M, Chung H-S, Lee K-J, Kim H-J (2000) J Anal Toxicol 24:238–244
Schachter M, Marsden C D, Parkes JD, Jenner P, Testa B (1980) J Neurol Neurosurg Psychiatry 43:1016–1021
Szöko É, Kalász H, Magyar K (1999) Eur J Drug Metab Pharmacokinet 24:315–319
Laine K, Anttila M, Huupponen R, Maki-Ikola O, Heinonen E (2000) Clin Neuropharmacol 23:22–27
Clement B, Behrens D, Möller W, Cashman JR (2000) Chem Res Toxicol 13:1037–1045
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at: 5th Balaton Symposium on High-Performance Separation Methods, Siofok, Hungary, September 3–5, 2003
Rights and permissions
About this article
Cite this article
Szöko, É., Tábi, T., Halász, A. et al. Chiral Characterization and Quantification of Deprenyl-N-oxide and Other Deprenyl Metabolites in Rat Urine by Capillary Electrophoresis. Chromatographia 60 (Suppl 1), S245–S251 (2004). https://doi.org/10.1365/s10337-004-0301-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-004-0301-1