Abstract
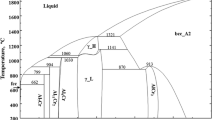
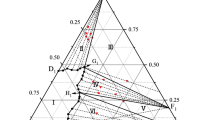
A thermodynamic assessment of the Al-Cu-Mg ternary system is presented. The Gibbs energies for the liquid and solid solution phases were modeled using the Redlich-Kister polynomial and the Wagner-Schottky model represented by the compound-energy formalism. The model parameters were obtained after fitting to previously critically assessed experimental phase diagram and thermodynamic data available in the literature. The thermodynamic functions and phase diagram calculated using the model parameters describe quite well the known experimental information. The complete set of Gibbs energies for all phases appearing in this system enables the calculation of thermodynamic values as a function of composition and temperature even for those ranges where no experimental information is available.
Similar content being viewed by others
Cited References
M.H.G. Jacobs and PJ. Spencer,J. Alloy. Comp. 220, 15 (1995).
H. Feufel, T. Gödecke, H.L. Lukas, and F. Sommer,J. Alloy. Compd, 247, 31 (1997).
S. Chen, Ph.D. thesis, University of Wisconsin-Madison (1994).
D. Soares, L.F. Malheiros, M. HÄmÄlÄinen, and F. Castro,J. Alloy. Compd., 220, 179 (1995).
Y.B. Kim, F. Sommer,and B. Predel,Z Metallkd., 86, 597 (1995).
A. Prince and G. Effenberg, Aluminium-Copper-Magnesium,Ternary Alloys, G. Petzow and G. Effenberg, Ed., Vol. 4, VCH Verlagsges., Weinheim, 547 (1991).
N. Saunders, private communication (1992).
N. Saunders,CALPHAD, 14,61 (1990), with revision by the author.
C.A. Coughanowr, I. Ansara, R. Luoma, M. HÄmÄlÄinen, and H.L. Lukas,Z Metallkd., 82, 574 (1991), with revision by the authors.
H.W. Phillips,J. Inst. Metals, 25, 27 (1959).
A.I. Beljaew,Metallovedenic Aluminija, Metallurgia, Moscow, 73 (1983).
Y.A. Chang, J.P. Neumann, A. Mikula, and D. Goldberg,Phase Diagrams and Thermodynamic Properties of Ternary Copper-Metal Systems, INCRA Monograph Series VI, 175(1979).
E.K. Rodionova, N.M. Martynova, and L.J. Cherneeva,Zh. Fiz. Khim., 6, 1382(1986).
Y. Zuo and Y. A. Chang,light Metals, S.K. Das, Ed., Minerals, Metals & Materials Society, 935 (1993).
C.C. Huang and S.W. Chen,Met. Mater. Trans., 26A, 1007 (1995).
B. Predel and H. Ruge,Mater. Sci. Eng., 9, 141 (1972).
D. Farkas and C.E. Birchenall,Metall. Trans. A, 16A, 323 (1985).
M. Notin, M. Dirand, D. Bouaziz, and J. Hertz,C.R. Acad. Sci. Paris, 302, 63 (1986).
M.I. Zamotonn,Lenin Pol. Inst., Trudy, 18032(1955).
D.A. Petrow and G.S. Berg,Zh. Fiz. Khim, 20, 1475 (1946).
G.G. Urazov and M.S. Mirgalovskaya,Izv. Sekt. Fiz. Khim. Anal., 79, 514(1949).
E.V. Melnik and V.V. Kinzhibalo,Russ.Metall., 3, 154(1981).
F. Laves and H. Witte,Metallwirtschaft, 15, 15–22 (1936).
T. Buhler, S.G. Fries, P.J. Spencer, and H.L. Lukas,J. Chim. Phys., 94, 1043 (1997).
G. Bergman, J.L.T. Waugh, and L. Pauling,Acta Crystallogr., 10, 254(1957).
P. Villars and L.D. Calvert,Pearson’s Handbook of Crystallographic Data for Intermetallic Phases, Vol. 1, ASM International, Materials Park, OH, 745 (1985).
I. Ansara,CODATA 94, Elsevier, Amsterdam (1996).
A.T. Dinsdale,Calphad, 15, 317 (1991).
O. Redlich and A. Kister,Ind. Eng. Chem., 40, 345 (1948).
Y.-M. Muggianu, M. Gambino,and J.-P. Bros,J. Chim. Phys., 72, 83 (1975).
C. Wagner,Thermodynamics of Alloys, Addison-Wesley, Cambridge, MA (1952).
I. Ansara, private communication.
P. Liang, H.L. Lukas, H.J. Seifert, and F. Aldinger, Proc. ofCAL-PHADXXVI, Palm Coast, 10, May 1997.
B. Sundman, B. Jansson, and J.-O. Andersson,Calphad, 9, 153 (1985).
H. Nishimura,Mem Coll. Eng., Kyoto Imp. Univ., 10, 18 (1937).
H. Nishimura,Mem Coll. Eng., Kyoto Imp. Univ., 10, 117(1938).
H. Hanemann and A. Schrader, Aluminium-Cupfer-Magnesium,TernÄre Legierungen des Aluminiums, Verlag Stahleisen, Düsseldorf, 73 (1952).
G.G. Urazov and D.A. Petrov,Zh. Fiz. Khim., 20, 387 (1946).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Buhler, T., Fries, S.G., Spencer, P.J. et al. A thermodynamic assessment of the Al-Cu-Mg ternary system. JPE 19, 317–333 (1998). https://doi.org/10.1361/105497198770342058
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1361/105497198770342058