Abstract
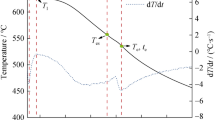
Solidification simulation of an aluminum-base multicomponent alloy was carried out by a method combining thermodynamic analysis using Thermo-Calc and heat-transfer calculation. An Al-9.5% Si-3% Cu-1% Mg-0.8% Fe (all mass%) aluminum-base multicomponent alloy was used for the simulation. The effect of latent heat on the heat-transfer calculation was considered by using an enthalpy method. The temperature-enthalpy curves for both an equilibrium state and nonequilibrium state with assumptions of no diffusion in the solid were calculated by using Thermo-Calc. A small casting with a cylindrical shape was used for the heat-transfer simulation. The vertical cross section of the casting was divided into rectangular grids, and the enthalpy change of each grid was numerically calculated. The calculated enthalpies in the grids were converted for each time-step into temperatures by using the temperature-enthalpy curve. A casting experiment was carried out under the same conditions as those of the simulation, and the calculated cooling curves obtained under the nonequilibrium condition agreed with the experimental ones.
Similar content being viewed by others
References
E.A. Mizikar: Trans. AIME, 1967, vol. 239, p. 1747.
I. Ohnaka and T. Fukusako: Trans. Iron Steel Inst. Jpn., 1977, vol. 17, p. 410.
I. Ohnaka: Introduction of Computer Analysis for Heat Transfer and Solidification, Maruzen, Tokyo, 1985, p. 167.
R.A. Roberts, R.P. Date, C.R. Loper, and D.R. Poirier: Trans. AFS, 1968, vol. 76, p. 573.
C.R. Loper, R.W. Heine, and R.A. Roberts: Trans. AFS, 1969, vol. 76, p. 373.
W. Koppe and S. Engler: Giesserei, 1962, vol. 49, p. 265.
W. Heine: AFS Trans., 1965, vol. 73, p. 34.
R.A. Johns: AFS Trans., 1980, vol. 88, p. 77.
B. Sundman, B. Jannson, and J.-O. Andersson: CALPHAD, 1985, vol. 9, p. 261.
S. Nagasaki: Data Book of Metals, Japan Institute of Metals, Maruzen, Tokyo, 1993, p. 1.
E. Scheil: Z. Metallkd., 1942, vol. 34, p. 70.
K. Ohsasa, M. Shoji, and T. Narita: J. Jpn. Foundry Eng. Soc., 2000, vol. 72, p. 525.
H.D. Brody and M.C. Flemings: Trans. TMS-AIME, 1966, vol. 236, p. 615
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ohsasa, K. Numerical simulation of solidification for aluminum-base multicomponent alloy. JPE 22, 498–503 (2001). https://doi.org/10.1361/105497101770333081
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1361/105497101770333081