Abstract
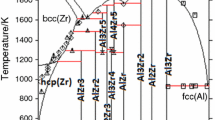
The thermodynamic parameters of the Al-Zr binary system can be very useful for the development of Zr-Al based amorphous and nanocrystalline materials. Phase diagram and thermochemistry data, especially the enthalpy of mixing of the liquid phase and the standard enthalpy of formation for the intermetallic compounds, are employed to optimize a consistent thermodynamic description for all the phases. The liquid, fcc, bcc, and hcp phases are treated as disordered solutions, while all the intermetallic phases are taken as stoichiometric compounds. The calculated phase diagram and thermochemistry data agree well with most of the experimental values reported in the literature.
Similar content being viewed by others
References
A. Inoue, T. Zhang, M.W. Chen, T. Sakurai, J. Saida, and M. Matsushita: J. Mater. Res., 2000, vol. 15, pp. 2195–2208.
A. Inoue, T. Zhang, and M. Matsushita: Mater. Trans. JIM, 1990, vol. 31, p. 177.
Ni-Data, Version 4.0, Thermo-Tech Ltd., Surrey, United Kingdom, 2000.
N. Saunders and V.G. Rivlin: Mater. Sci. Technol., 1986, vol. 2, pp. 521–27.
N. Saunders: Z. Metallkd., 1989, vol. 80, pp. 894–903.
A. Peruzzi: J. Nucl. Mater., 1992, vol. 186, pp. 89–99.
S.V. Meschel and O.J. Kleppa: J. Alloy Compounds, 1993, vol. 191, pp. 111–16.
R. Klein, I. Jacob, P.A.G. O’Hare, and R.N. Goldberg: J. Chem. Thermodyn., 1994, vol. 26, pp. 599–608.
J. Murray, A. Peruzzi, and J.P. Abriata: J. Phase Equilibria, 1992, vol. 13, pp. 277–91.
H. Okamoto: J. Phase Equilibria, 1993, vol. 14, pp. 259–60.
D.J. McPherson and M. Hansen: Trans. ASM, 1954, vol. 46, pp. 354–74.
S.N. Tiwari and K. Tangri: J. Nucl. Mater., 1970, vol. 34, pp. 92–96.
E.M. Schulson, D.H. McColl, and V.C. Ling: Report No. AECL-5176, Atomic Energy of Canada Limited, Chalk Review Laboratories, Chalk River, Canada, July 1975, Cited in Ref 6.
R.J. Kematick and H.F. Franzen: J. Solid State Chem., 1984, vol. 54, pp. 226–34.
W.L. Fink and L.A. Willey: Met. Technol., 1939, vol. 1, pp. 69–80.
V.M. Glazov, G. Lazarev, and Korolkov: Metalloved. Term. Obrab. Met., 1959, vol. 10, pp. 48–50.
M.E. Drits, E.S. Kadaner, and V.I. Kuz’mina: Izv. Akad. Nauk SSSR, 1968, vol. 1, pp. 102–05.
G.M. Kuznetsov, A.D. Barsukov, and M.I. Abas: Sov. Non-Ferrous Met. Res., 1983, vol. 11 (1), pp. 47–51.
P. Chiotti and P.F. Woerner: J. Less-Common Met., 1964, vol. 7, pp. 111–19.
Y.O. Esin, N.P. Bobrov, M.S. Petrushevskiii, and P.V. Gel’d: Izv. Akad. Nauk SSSR, Met., 1974, vol. 5, pp. 104–09.
V.S. Sudavtsova, G.I. Batalin, and V.S. Tutevich: Izv. Akad. Nauk SSSR, Met., 1985, vol. 5, pp. 185–87.
V. Witusiewicz, U.K. Stolz, I. Arpshofen, and F. Sommer: Z. Metallkd., 1998, vol. 89, pp. 704–13.
G.I. Batalin, E.A. Beloborodova, V.V. Nerubaschenko, V.D. Galochka, and L.I. Slyuzko: Izv. Vyssh. Ucheb. Zaved. Tsvetn. Metall., 1982, vol. 3, pp. 74–77.
Y.O. Esin, N.N. Serebrennikov, E.D. Pletneva, and V.K. Kapustkin: Izv. Vyssh. Ucheb. Zaved., Chern. Metall., 1987, vol. 10, pp. 1–3.
A.T. Dinsdale: CALPHAD, 1991, vol. 15, pp. 317–425.
B. Sundman, B. Jansson, and J.O. Anderson: CALPHAD, 1985, vol. 9, pp. 153–90.
M. Potzschke and K. Schubert: Z. Metallkd., 1962, vol. 53 (8), pp. 548–61.
C.B. Alcock, K.T. Jacob, and S. Zador: in Zirconium: Physico-Chemical Properties of its Compounds, Atomic Energy Review No. 6, O. Kubaschewski, ed., IAEA, Vienna, 1976.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wang, T., Jin, Z. & Zhao, JC. Thermodynamic assessment of the Al-Zr binary system. JPE 22, 544–551 (2001). https://doi.org/10.1361/105497101770332695
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1361/105497101770332695