Abstract
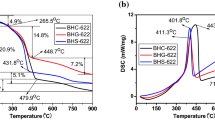
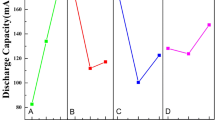
The synthesis process of LiCo0.3Ni0.7O2 was investigated by FT-IR, mass spectroscopy, elemental analysis, SEM, BET, TG/DTA and XRD in this paper. The results revealed that lithium and transition metal ions were trapped homogeneously on an atomic scale throughout the precursor. Li2CO3, NiO and CoO are the intermediate products obtained after decomposition of the precursor and Li2CO3 undergoes direct reactions with NiO and CoO to form LiCo0.3Ni0.7O2. Moreover, the kinetics of formation of LiCo0.3Ni0.7O2 by citrate sol-gel method is faster than the case of the conventional solid-state reaction between lithium carbonate and corresponding reactants. The single phase of LiCo0.3Ni0.7O2 was synthesized at temperature as low as 550°C. The discharge capacity of LiCo0.3Ni0.7O2 increases from 127 to 185 mAh/g as the calcination temperature increasing from 550 to 750X2. After 100 cycles, the discharge capacity of the sample calcined at 750°C is 155 mAh/g. The electrochemical study shows that the LiCo0.3Ni0.7O2 has high discharge capacity and good cycling behavior for lithium ion batteries.
Similar content being viewed by others
References
Chen, Z. H., Dahn, J. R., Effect of a ZrO2 coating on the structure and electrochemistry of LixCoC2 when cycled to 4.5 V, Electrochem. Solid-State Lett., 2002, 5(10): A213-A216.
Castro-Garcia, S., Castro-Couceiro, A., Senaris-Rodriguez, M. A. et al., Influence of aluminum doping on the properties of LiCoO2 and LiCo0.5Ni0.5O2 oxides, Solid State Ionics, 2003, 156: 15–26.
Hwang, B. J., Santhanam, R., Chen, C. H., Effect of synthesis conditions on electrochemical properties of LiNi1−yCoyO2 cathode for lithium rechargeable batteries, J. Power Sources, 2003, 114: 244–252.
Sheu, S. P., Shih, I. C., Yao, C. Y. et al., Studies of LiNiO2 in lithium-ion batteries, J. Power Sources, 1997, 68: 558–560.
Kim, J., Amine, K., A comparative study on the substitution of divalent, trivalent and tetravalent metal ions in LiNi1−xMxO2 (M=Cu2+, Al3+ and Ti4+), J. Power Sources, 2002, 104: 33–39.
Caurant, D., Baffler, N., Garcia, B. et al., Synthesis by a soft chemistry route and characterization of LiNixCo1-xO2 (0⩽x1) cathode materials, Solid State Ionics, 1996, 91: 45–54.
Wang, G. X., Horvat, J., Bradhurst, D. H. et al., Structural, physical and electrochemical characterisation of LiNixCo1−xO2 solid solutions, J. Power Sources, 2000, 85: 279–283.
Cho, J., Jung, H. S., Park, Y. C. et al., Electrochemical properties and thermal stability of LiaNi1−xCoxO2 cathode materials, J. Electrochem. Soc, 2000, 147(1): 15–20.
Cho, J., Kim, G., Lim, H. S. et al., Effect of preparation methods of LiNi1−xCoxO2 cathode materials on their chemical structure and electrode performance, J. Electrochem. Soc, 1999, 146: 3571–3576.
Madhavi, S., Subba Rao, B. V., Chowdari, B. V. R. et al., Effect of aluminum doping on cathodic behaviour of LiNi0.7Co0.3O2, J. Power Sources, 2001, 93: 156–162.
Subba Rao, G. V., Chowdari, B. V. R., Lindner H. J., Yttrium-doped Li (Ni, Co)O2: an improved cathode for Li-ion batteries, J. Power Sources, 2001, 97-98: 313–315.
Fey, G. T. K., Subramanian, V., Lu, C. Z., Tartaric acid-assisted sol-gel synthesis of LiNi0.8Co0.2O2 and its electrochemical properties as a cathode material for lithium batteries, Solid State Ionics, 2002, 152-153: 83–90.
Fey, G. T. K., Shiu, R. F., Subramanian, V. et al., LiNi0.8Co0.2O2 cathode materials synthesized by the maleic acid assisted sol-gel method for lithium batteries, J. Power Sources, 2002, 103: 265–272.
Moshtev, R., Zlatilova, P., Bakalova, I. et al., synthesis, XRD characterization, and cycling performance of cobalt doped lithium nickelates, J. Power Sources, 2002, 112: 30–35.
Suresh, P., Rodrigues, S., Shukla, A. K. et al., synthesis of LiCo1−xNixO2 from a low temperature solution combustion route and characterization, J. Power Sources, 2002, 112: 665–670.
Gover, R., Kanno, R., Mitchell, B. et al., The effects of sintering time on the structure and electrochemical properties of Li (Ni0.8Co0.2)O2, J. Power Sources, 2000, 90: 82–88.
Ying, J. R., Wan, C. R., Jiang, C. Y. et al., Preparation and characterization of high-density spherical LiNi0.8Co0.2O2 cathode material for lithium secondary batteries, J. Power Sources, 2001, 99: 78–84.
Jing, D. M., Zhu, W. X., Approach of Coordination Chemistry (in Chinese), Beijing: Science Press, 1996, 514–521.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tong, D., Lai, Q., Lu, J. et al. Synthesis of LiCo0.3Ni0.7O2 as cathode materials for lithium ion batteries by citric acid-assisted sol-gel method. Chin.Sci.Bull. 50, 1087–1093 (2005). https://doi.org/10.1360/04wb0111
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1360/04wb0111