Abstract
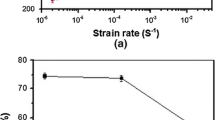
The role of atomic hydrogen and hydrogen-induced martensites in hydrogen embrittlement in slow strain rate tensile tests and hydrogen-induced delayed cracking (HIC) in sustained load tests for type 304 L stainless steel was quantitatively studied. The results indicated that hydrogen-induced martensites formed when hydrogen concentration C 0 exceeded 30 ppm, and increased with an increase in C 0, i.e. M(vol%)=62–82.5 exp (−C 0/102). The relative plasticity loss caused by the martensites increased linearly with increasing amount of the martensites, i.e. l δ(M), %=0.45 M (vol %)=27.9−37.1 exp(−C0/102). The plasticity loss caused by atomic hydrogen l δ(H) increased with an increase in C 0 and reached a saturation value l δ(H)max=40% when C 0>100 ppm. l δ(H) decreased with an increase in strain rate \(\dot \varepsilon \), i.e. l δ(H), \(\% = - 21.9 - 9.9\dot \varepsilon \), and was zero when \(\dot \varepsilon \geqslant \dot \varepsilon _c = 0.032/s\). HIC under sustained load was due to atomic hydrogen, and the threshold stress intensity for HIC decreased linearly with in C 0, i.e. K IH (Mpam1/2)=91.7−10.1 In C 0 (ppm). The fracture surface of HIC was dimple if K 1 was high or/and C 0 was low, otherwise it was quasi-cleavage. The boundary line between ductile and brittle fracture surface was K 1-54+25exp(−C 0/153)=0.
Similar content being viewed by others
References
Theus, G. J., S., Review of SCC and HE in a austenitic Fe−Cr−Ni alloys, in SCC and HE of Iron Base Alloys (eds. Staehle, R., Hochmann, J., McCright, R., et al.), NACE, Texas Hoaston, 1977, 845–897.
Holzworth, M. L., Louthan, M. R., Hydrogen-induced phase transformations in type 304L stainless steels, Corrosion, 1968, 24: 110–124.
Perng, T. P., Altstetter, C. J., Effects of water vapor on hydrogen induced slow crack growth in stainless steels, Metall. Trans. A, 1988, 19A: 651–656.
Chu, W. Y., Wang, H. L., Hsiao, C. M., The mechanism of slow crack growth and SCC in stainless steel, Corrosion, 1984, 40: 487–492.
Narita, N., Birnbaum, H., On the role of phase transitions in the hydrogen embrittlement of stainless steels, Scripta Metall., 1980, 14: 1355–1358.
Sing, S., Altstetter, C., Effect of hydrogen concentration on slow crack growth in stainless steels, Metall. Trans. 1982, 13A: 1799–1801.
Huang, T. H., Altstetter, C. J., Internal hydrogen-induced suberitical crack growth in stainless steels, Metall. Trans. A, 1991, 22A: 2605–2627.
Brooks, J. A., West, A. J., Hydrogen induced ductility losses in austenitic stainless steel welds, Metall. Trans. A, 1981, 12A: 213–223.
Chu, W. Y., Yao, J., Hsiao, C. M., Hydrogen induced slow crack growth in stable austenitic stainless steel, Metall. Trans. A, 1980, 15A: 729–733.
Qiao, L. J., Chu, W. Y., Hsiao, C. M., SCC and hydrogen-induced cracking in austenitic stainless steel under mode III loading, Corrosion, 1987, 43: 479–484.
Qiao, L. J., Chu, W. Y., Hsiao, C. M., SCC and hydrogen-induced cracking in austenitic stainless steel under mode II loading, Corrosion, 1988, 44: 50–55.
Hanninen, H., Hakarainer, T., On the effect of α′ martensite in HE of stainless steels, Corrosion, 1980, 36: 47–51.
Yu, G. H., Cheng, Y. H., Chen, L. J. et al., Hydrogen accumulation and HIC of API C90 tubular steel, Corrosion, 1997, 53: 762–769.
Zhang, T. Y., Chu, W. Y., Hsiao, C. M., Tetragonal distortion field of hydrogen atoms in iron, Metall. Trans. A, 1985, 16A: 1649–1653.
Chu, W. Y., Hsiao, C. M., Zhao, X. Z., Corrosion fatigue crack initiation in mode II notch specimen, Metall. Trans. A, 1988, 19A: 1067–1073.
Lu, H., Li, M. D., Zhang, T. Z. et al., Hydrogen-enhanced dislcoation emission, motion and nucleation of HIC for steel, to be published.
Bai, X. Z., Chu, W. Y., Hsiao, C. M., Partial molar strain field of hydrogen in α-Fe, Scripta Metall., 1987, 21: 613–618.
Leeuwen, H. P., The kinetics of hydrogen embrittlement: a quantitiative diffusion model, Eng. Frac. Mech., 1974, 6: 141–161.
Li, J. C. M., Physical chemistry of some microstructural phenomena, Metall. Trans. A, 1978, 9A: 1353–1380.
Li, J. C. M., Computer simulation of dislocations emitted from a crack, Scripta Metall., 1986, 20: 1447–1482.
Zhang, T. Y., Chu, W. Y., Hsiao, C. M., Mecchanism of hydrogen-induced softening, Sci. in China, Ser. A, 1986, 29: 1157–1171.
Birnbaum, H. K., Sofronis, P., Hydrogen enhanced localization plasticity, Mater. Sci. Eng., 1994, A176: 191–202.
Abraham, D. P., Altstetter, C. J., Hydrogen-enhanced localization of plasticity in an austenitic stainless steel, Metall. Mater. Trans., 1995, 26A: 2859–2871.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pan, C., Wuyan, C., Zhengbang, L. et al. The role of atomic hydrogen and hydrogen-induced martensites in hydrogen embrittlement of type 304L stainless steel. Sci. China Ser. E-Technol. Sci. 45, 175–183 (2002). https://doi.org/10.1360/02ye9022
Received:
Issue Date:
DOI: https://doi.org/10.1360/02ye9022