Abstract
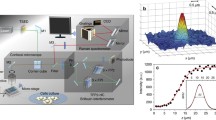
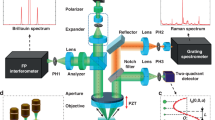
Cells adhesion is very important for many physiological processes. Using advanced Raman microspectroscopic technique, we selected T Leukemia cells (Jurkat) as the materials and obtained simultaneously conformation information of various biomolecules inside the whole living cells. By comparing the Raman microspectroscopic spectra of single and adhesive cancer cells, we found for the first time that when cells adhered, the conformation of the biomolecules (DNA, protein, carbohydrates and lipids) inside the cells had different changes: (i) the backbone of double-stranded DNA maintained orderly B-form or modified B-form conformation, whereas the groups of its deoxyribose and bases were modified; (ii) the conformational changes of the main chain and the side chain in the protein were obviously variant. The lines intensity belonging to α-helix and β-sheet decreased, while that of β-turn increased. Tyrosine and tryptophane residues of the protein changed from “buried state” to “exposed state”; the lines intensity of its sulfhydryl group also increased; the conformation of its disulfide bond changed from two kinds to three kinds. These facts suggest that the cells adhesion causes changes in H-bonds organization of the main chain and environment of the side chain in the protein; (iii) the groups of the carbohydrates were also modified simultaneously; (iv) the conformation of the lipids bilayers of the membranes changed obviously; the order parameter for lateral interaction between chains decreased gradually with the increase of number of the adhesive cells. So cells adhesion resulted in an increase in fluidity of the membrane and ion permeability on the membrane.
Similar content being viewed by others
References
Trinkaus, J. P., Cells into Organs: The Forces that Shape the Embryo, 2nd ed., New Jersey: Prentice-Hall Inc., 1984, 69–71.
Maheshwari, G., Wells, A., Griffith, L.G. et al., Biophysical integration of effects of epidermal growth factor and fibronectin on fibroblast migration, Biophy. J., 1997, 76: 2814–2823.
Neelamegham, S., Munn, L. L., Zygourakis, K., A model for kinetics of homotypic cellular aggregation under static conditions, Biophy. J., 1997, 72: 51–64.
Lampugnani, M. G., Dejana, E., Interendothelial junctions: structure, signalling and functional roles, Curr. Opin. Cell Biol., 1997, 9: 674–682.
Vasioukhin, V., Christoph, B., Mei, Y. et al., Directed actin polymerization is the driving force for epithelial cell-cell adhesion, Cell, 2000, 100: 209–219.
Milier, J. R., Moon, R. T., Signal transduction though β-catenin and specification of cell fate during embryogenesis, Genes Dev., 1996, 10: 2527–2539.
Peifer, M., Cell adhesion and signal transduction: the armadillo connection, Trends Cell Biol., 1995, 5: 224–229.
Gumbiner, B. M., Cell adhesion: the molecular basis of tissue architecture and morphogenesis, Cell, 1996, 84: 345–357.
Gurdon, G. B., A community effect in animal development, Nature, 1983, 336: 772–774.
Zhuang, X. H., Cell sociology, in The Frontiers of Life Science Facing the 21th Century. (eds. Li, B. J. et al.), Guangdong: Guangdong Sci. and Tech. Press, 1996, 279–290.
Terzaghi-Howe, M., Inhibition of carcinogen-altered rat trachea epithelial cells proliferation by normal epithelial cells in vivo, Carcinogenesis, 1987, 8: 145–150.
Cal, C., Freije, J. M. P., Lopez, J. M. et al., ADAM 23/MDC3, a human disintegrin that promotes cell adhesion via interaction with the alpha v beta 3 integrin through an RGD-independent mechanism, Mole. Biol. Cell, 2000, 11: 1457–1469.
Baumgartner, W., Hinterdorfer, P., Ness, W. et al., Cadherin interaction probed by atomic force microscopy, Proc. Natl. Acad. Sci. USA, 2000, 97: 4005–4010.
Wilkemeyer, W. F., Sebastian, A. B., Smith, S. A. et al., Antagonists of alcohol inhibition of cell adhesion, Proc. Natl. Acad. Sci. USA, 2000, 97: 3690–3695.
Greve, J., Puppels, G. J., Raman microspectroscopy of single whole cells, in Biomolecular Spectroscopy (eds. Clark, R. J. H., Hester, R. E.), Part A, Vol. 20, London: Wiley, 1993, 231–265.
Goodwin, D. C., Brahms, J., Form of DNA and the nature of interactions with protein in chromatin, Nucleic Acids Res., 1978, 5(3): 835–850.
Xu, Y. M., Zhou, Z. X., Yang, H. Y. et al., Raman spectroscopic study of microcosmic photodamage of the space structure of DNA sensitized by Yangzhou haematoporphyrin derivative and photofrin II, Int. J. Photochem & Photobiol., B: Biol., 1999, 52: 30–34.
Thomas, G. J. Jr., Kyogoku, Y., Biological science, in Infrared and Raman Spectroscopy (eds. Bram, E. G., Grasselli, J. G.), Part C, Maryland: Marcel Dekker, Inc., 1977, 717–872.
Carey, P. R., Biochemical Applications of Raman and Resonance Raman Spectroscopies, New York: A Subsidiary of Harcourt Brace Jovanovich Publishers, 1982, 77–90.
Lord, R.C., Yu, N. T., Laser-excited Raman spectroscopy of biomolecules (I) —Native lysozyme and constituent Amino acids, J. Mol. Biol., 1970, 50: 509–524.
Krimm, S., Bandekar, J., Vibrational analysis of peptides, polypeptides, and proteins (V) —Normal vibrations of β-turns, J. Biopolymers, 1980, 19: 1–29.
Bandekar, J., Krimm, S., Vibrational analysis of peptides, polypeptides, and proteins (VI) —Assignment of β-turns Modes in insulin and other proteins, J. Biopolymers, 1980, 19: 31–36.
Xu, Y. M., Yang, H. Y., Zhang, Z. Y., Raman spectroscopic study of space structure of membrane proteins and membrane lipids in photodamaged human erythrocyte sensitized by hypocrellin B, Science in China, Ser. C, 1998, 41(6): 608–616.
Wu, C., Xu, Y. M., Fan, R., Interaction between fibrinokinase l-TPA and human fibrin containing profibrinolysin, Kexue-Tongbao, 1988, 33(7): 599–601.
Alberts, B., Bray, D., Lewis, J. et al., Molecular Biology of the Cell, 2nd ed., New York: Garland Publishing, Inc., London, 1989, 1–15.
Ichikawa, Y., Lin, Y.C., Dumas, D.P. et al., Chemical-enzymatic synthesis and conformational analysis of sialyl Lewis × and derivatives, J. Am. Chem., 1992, 114: 9283–9298.
She, C.Y., Dinh, N. D., Tu, A. T., Laser Raman scattering of glucosamine, N-acetylglucosamine and glucuronic acid, Biochim. Biophys. Acta, 1974, 372: 345–357.
Zhao, H. X., Xu, Y. M., Lu, C. Z., Raman spectroscopic study of D-Mannose after the photosensitive damage caused by hypericin, Asian J. Spectroscopy, 1997, 1: 71–76.
Tu, A. T., Raman Spectroscopy in Biology: Principles and Applications, New York: John Wiley, 1982, 236–240.
Gaber, B. P., Peticalas, W. L., On the quantitative interpretation of biomembrane structure by Raman spectroscopy, Biochim. Biophys. Acta, 1977, 465: 260–274.
Xu, Y. M., Zhao, H. X., Zhang, Z. Y., Raman spectroscopic study of microcosmic and photosensitive damage on the liposomes of the mixed phospholipids sensitized by hypocrellin and its derivatives, Int. J. Photochem. & Photobiol., B: Biol., 1998, 43: 41–46.
Xu, Y. M., Yang, H. Y., Yan, Y. C. et al., Raman spectroscopic study of photodamage on the space structure of DL-α-phosphatidylcholine liposomes sensitized by hypocrellin B, Int. J. Photochem. & Photobiol. B: Biol., 1998, 45: 179–183.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, G., Yang, H., Xu, Y. et al. Raman microspectroscopic study of biomolecular structure inside living adhesive cells. Sci. China Ser. C.-Life Sci. 45, 397–405 (2002). https://doi.org/10.1360/02yc9044
Received:
Issue Date:
DOI: https://doi.org/10.1360/02yc9044