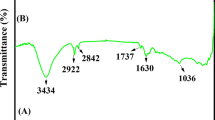
Abstract
A novel determination method of electroinactive molecules by means of electrochemical technique is presented. A new self-assembled monolayer containing cyclodextrin (CD) is prepared with mono(6-o-p-tolylsulfonyl)-β-cyclodextrin. Although this derivatization process leads to a β-CD coverage of 10% of a full monolayer, this layer shows an effective host-guest response to ferrocene. The interfacial ferrocene complexation gives a response similar to that expected for a Langmuir adsorption isotherm yielding a stability constant of 4.2×104 mol-1.L and a maximum ferrocene coverage of 8.6×10-12 mol/cm2. The redox peak currents of the surfaceconfined ferrocene decrease upon addition of competing β-CD guest species to the solution, such as m-toluic acid (mTA) and sodium dodecyl sulfonate (SDS). This principle has been used for the determination of the electroinactive molecules, mTA and SDS in the concentration ranges of 0.8-2.7 μmol/L and 5–100 nmol/L, respectively.
Similar content being viewed by others
References
Dubois, L. H., Nuzzo, R. G., Synthesis, structure and properties of model organic surfaces, Annu. Rev. Phys. Chem., 1992, 43: 437–463.
Abbott, N. L., Whitesides, G. M., Potential-dependent wetting of aqueous solutions on self-assembled monolayers formed from 15-(ferrocenylcarbonyl)pentadecanethoil on gold, Langmuir, 1994, 10: 1493–1497.
Liu A. C., Chen D. C., Lin, C. C. et al., Application of cysteine monolayers for electrochemical determination of sub-ppb copper(II), Anal. Chem.,1999, 71: 1549–1552.
Malem, F., Mandler, D., Self-assembled monolayers in electroanalytical chemistry: application of ω-mercapto carboxylic acid monolayers for the electrochemical detection of dopamine in the presence of a high concentration of ascorbic acid, Anal. Chem., 1993, 65: 37–41.
Sun, L., Crooks, R. M., Ricco, A. J., Molecular interactions between organized, surface-confined monolayers and vapor-phase probe molecules 5: acid-base interactions, Langmuir, 1993, 9: 1775–1780.
Kitano, H., Taira, Y., Tamamoto, H., Inclusion of phthalate esters by a self-assembled monolayer of thiolated cyclodextrin on a gold electrode, Anal. Chem., 2000, 72: 2976–2980.
Yan, J., Dong, S. J., Formation of surface inclusion complexes between cyclodextrins and n-alkanethiols and their self-assembled behaviors on gold, Langmuir, 1997, 13: 3251–3255.
Ju, H. X., Leech, D., Host-guest interaction at SAM/solution interface: an electrochemical analysis on the inclusion of 11-(ferrocenylcarbonyloxy)undecane thiol on Au by cyclodextrin, Langmuir, 1998, 14: 300–306.
Rojas, M. T., Koniger, R., Stoddart, J. F. et al., Supported monolayers containing preformed binding sites, Synthesis and interfacial binding properties of a thiolated β-cyclodextrin derivative, J. Am. Chem. Soc., 1995, 117: 336–343.
Bermardo, A. R., Lu, T., Cordova, E. et al., Host-guest complexation at the electrode/solution interface: the inclusion of an amphiphilic viologen guest by an amphiphilic calix[6]arene host, J. Chem. Soc., Chem. Commun., 1994: 529-530.
Kaifer, A. E., Interplay between molecular recognition and redox chemistry, Acc. Chem. Res., 1999, 32: 62–71.
Wang, J., Liu, J., Calixarene-coated amperometric detectors, Anal. Chim. Acta, 1994, 294: 201–206.
Chamberlain II, R. V., Slowinska, K., Majda, M., Electrostatically-induced inclusion of anions in cyclodextrin monolayers on electrodes, Langmuir, 2000, 16: 1388–1396.
Maeda, Y., Kitano, H., Inclusional complexation by cyclodextrins at the surface of silver as evidenced by surface-enhanced resonance Raman spectroscopy, J. Phys. Chem., 1995, 99: 487–488.
Odashima, K., Kotato, M., Sugawara, M. et al., Voltammetric study on a condensed monolayer of a long alkyl cyclodextrin derivative as a channel mimetic sensing membrane, Anal. Chem., 1993, 65: 927–936.
Hamasaki, K., Ikeda, H., Nakamura, A. et al., Fluorescent sensors of molecular recognition: modified cyclodextrins capable of exhibiting gust-responsive twisted intramolecular charge transfer fluorescence, J. Am. Chem. Soc., 1993, 115: 5035–5040.
Li, Y. M., Qi, W. B., Chen, Y. H., Investigation on effect of β-cyclodextrin on chromatic reaction, II. Discussion on coordinative action mechanism with surfactants, Chinese J. Anal. Chem., 1994, 22: 548–551.
Wenz, G., Cyclodextrins as building blocks for supramolecular structures and functional units, Angew Chem. Int. Ed. Engl., 1994, 33: 803–822.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huangxian, J., Zong, D. & Leech, D. Electrochemical determination of electroinactive guests of β-cyclodextrin at a self-assembled monolayer interface. Sc. China Ser. B-Chem. 45, 46–53 (2002). https://doi.org/10.1360/02yb9007
Received:
Issue Date:
DOI: https://doi.org/10.1360/02yb9007