Abstract
Background
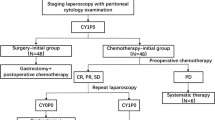
The significance of surgical resection in pancreatic ductal adenocarcinoma (PDAC) with positive peritoneal cytology (PPC) is controversial. This study aimed to evaluate whether preceding chemotherapy could be beneficial for patients with PDAC with PPC.
Methods
Between 2017 and 2019, 34 consecutive PDAC patients diagnosed with PPC without distant metastasis were retrospectively reviewed. Twenty-three patients did not receive neoadjuvant treatment (NAT) and 11 received NAT. All patients received systemic chemotherapy after PPC was confirmed, and they underwent surgical resection if PPC turned negative. The treatment course, ratio of conversion surgery (CS), and prognosis were evaluated. Moreover, the prognosis of PPC patients who underwent up-front surgery without NAT between 2003 and 2016 was analyzed as a comparative cohort.
Results
The median survival time (MST) of the patients without NAT was 31.4 months. CS was performed in 52.2% of the patients. Patients who underwent CS had better prognoses than those who did not undergo CS (p = 0.005). The CS rate was significantly higher in resectable PDAC (78.5%) than in borderline/unresectable PDAC (11.1%) (p = 0.002). The prognosis of patients with resectable PDAC was improved with preceding chemotherapy compared with up-front surgery (MST 13.0 months; p = 0.016). After NAT, the CS rate was low (27.3%), and the MST was only 14.1 months.
Conclusions
As an initial treatment for PDAC patients with PPC, chemotherapy may lead to a favorable prognosis. Especially, resectable PDAC is associated with a greater chance of improved prognosis. Future studies are required to ascertain whether up-front surgery or preceding chemotherapy should be performed for these patients.



Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30.
Cancer Registry and Statistics. Cancer Information Service NCC, Japan. (2020). https://ganjoho.jp/reg_stat/statistics/dl/index.html. Accessed 1 Nov 2020.
Tanaka M, Mihaljevic AL, Probst P, et al. Meta-analysis of recurrence pattern after resection for pancreatic cancer. Br J Surg. 2019;106:1590–601.
Ariake K, Motoi F, Ohtsuka H, et al. Predictive risk factors for peritoneal recurrence after pancreatic cancer resection and strategies for its prevention. Surg Today. 2017;47:1434–42.
Ariake K, Motoi F, Shimomura H, et al. 18-Fluorodeoxyglucose positron emission tomography predicts recurrence in resected pancreatic ductal adenocarcinoma. J Gastrointest Surg. 2018;22:279–87.
American Joint Committee on Cancer. AJCC Cancer Staging Manual. 8th edn. Chicago: Springer; 2017.
Brierley JD, Gospodarowicz MK, Wittekind C. Union for international cancer control. TNM classification of malignant tumours. 8th edn. Oxford: Wiley Blackwell; 2017.
Japan Pancreas Society. Classification of . English. Tokyo: Kanehara and Co., Ltd; 2017.
Satoi S, Murakami Y, Motoi F, et al. Reappraisal of peritoneal washing cytology in 984 patients with pancreatic ductal adenocarcinoma who underwent margin-negative resection. J Gastrointest Surg. 2015;19:6–14; discussion 14.
Tsuchida H, Fujii T, Mizuma M, et al. Prognostic importance of peritoneal washing cytology in patients with otherwise resectable pancreatic ductal adenocarcinoma who underwent pancreatectomy: a nationwide, cancer registry-based study from the Japan Pancreas Society. Surgery. 2019;166:997–1003.
Cao F, Li J, Li A, Li F. Prognostic significance of positive peritoneal cytology in resectable pancreatic cancer: a systemic review and meta-analysis. Oncotarget. 2017;8:15004–13.
Yin Z, Ma T, Chen S. Intraoperative peritoneal washing cytology on survival in pancreatic ductal adenocarcinoma with resectable, locally advanced, and metastatic disease. Pancreas. 2019;48:519–25.
National Comprehensive Cancer Network. NCCN practice guidelines for pancreatic cancer, Version 1. (2020). https://www.nccn.org/professionals/physician_gls/PDF/pancreatic.pdf. Accessed 1 Nov 2020.
Yamada S, Fujii T, Kanda M, et al. Value of peritoneal cytology in potentially resectable pancreatic cancer. Br J Surg. 2013;100:1791–6.
Oh SY, Edwards A, Mandelson MT, et al. Localized pancreatic cancer with positive peritoneal cytology as a sole manifestation of metastatic disease: a single-institution experience. Am J Surg. 2017;213:94–9.
Satoi S, Yamaue H, Kato K, et al. Role of adjuvant surgery for patients with initially unresectable pancreatic cancer with a long-term favorable response to non-surgical anti-cancer treatments: results of a project study for pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2013;20:590–600.
Ariake K, Motoi F, Mizuma M, et al. Locally advanced pancreatic cancer successfully treated by distal pancreatectomy with celiac axis resection (DP-CAR) after S-1 with radiation therapy followed by gemcitabine/nab-paclitaxel therapy: a case report. Surg Case Rep. 2017;3:15.
Satoi S, Fujii T, Yanagimoto H, et al. Multicenter phase II study of intravenous and intraperitoneal paclitaxel with S-1 for pancreatic ductal adenocarcinoma patients with peritoneal metastasis. Ann Surg. 2017;265:397–401.
Satoi S, Yanagimoto H, Yamamoto T, et al. Survival benefit of intravenous and intraperitoneal paclitaxel with S-1 in pancreatic ductal adenocarcinoma patients with peritoneal metastasis: a retrospective study in a single institution. J Hepatobiliary Pancreat Sci. 2017;24:289–96.
Mitachi K, Ariake K, Motoi F, Unno M. Conversion surgery for positive peritoneal washing cytology in pancreatic cancer. BMJ Case Rep. 2019;12:e229993.
Takadate T, Morikawa T, Ishida M, et al. Staging laparoscopy is mandatory for the treatment of pancreatic cancer to avoid missing radiologically negative metastases. Surg Today. 2020. https://doi.org/10.1007/s00595-020-02121-4.
Yoshioka R, Saiura A, Koga R, et al. The implications of positive peritoneal lavage cytology in potentially resectable pancreatic cancer. World J Surg. 2012;36:2187–91.
Yachida S, Fukushima N, Sakamoto M, Matsuno Y, Kosuge T, Hirohashi S. Implications of peritoneal washing cytology in patients with potentially resectable pancreatic cancer. Br J Surg. 2002;89:573–8.
Abe T, Ohuchida K, Endo S, et al. Clinical importance of intraoperative peritoneal cytology in patients with pancreatic cancer. Surgery. 2017;161:951–8.
Aoki S, Mizuma M, Hayashi H, et al. Prognostic impact of intraoperative peritoneal cytology after neoadjuvant therapy for potentially resectable pancreatic cancer. Pancreatology. 2020. https://doi.org/10.1016/j.pan.2020.08.022.
Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388:248–57.
Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–406.
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25.
Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703.
Acknowledgment
This study was supported by a Grant from the Pancreas Research Foundation of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Fuyuhiko Motoi—received research funding from Taiho pharm and Chugai Pharm. Michiaki Unno—Taiho, Takeda, Chugai, Yakult, Asahi Kasei.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ariake, K., Mizuma, M., Motoi, F. et al. Preceding Systemic Chemotherapy for Patients with Pancreatic Ductal Adenocarcinoma with Positive Peritoneal Cytology Provides Survival Benefit Compared with Up-Front Surgery. Ann Surg Oncol 28, 6246–6254 (2021). https://doi.org/10.1245/s10434-021-09718-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-09718-0