Abstract
Background
While combination therapy with nab-paclitaxel/gemcitabine (nab-gem) is effective in pancreatic ductal adenocarcinoma (PDAC), its efficacy as perioperative chemotherapy is unknown. The primary objective of this multicenter, prospective, single-arm, phase II study was to determine whether neoadjuvant therapy with nab-gem was associated with higher complete resection rates (R0) in resectable PDAC, while the secondary objectives were to determine the utility of radiological assessment of response to preoperative chemotherapy and the safety and efficacy of nab-gem as perioperative therapy.
Methods
Patients were recruited from eight Australian sites, and 42 patients with radiologically defined resectable PDAC and an Eastern Cooperative Oncology Group performance status of 0–2 were enrolled. Participants received two cycles of preoperative nab-paclitaxel 125 mg/m2 and gemcitabine 1000 mg/m2 on days 1, 8, and 15 (28-day cycle) presurgery, and four cycles postoperatively. Early response to chemotherapy was measured with fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) scans on day 15.
Results
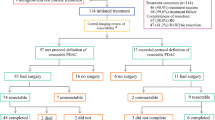
Preoperative nab-gem was completed by 93% of participants, but only 63% postoperatively. Thirty-six patients had surgery: 6 (17%) were unresectable, 15 (52%) had R0 (≥ 1 mm) resections, 14 (48%) had R1 (< 1 mm) resections, and 1 patient did not have PDAC. Median progression-free survival was 12.3 months and median overall survival (OS) was 23.5 months: R0 patients had an OS of 35 months versus 25.6 months for R1 patients after surgery. Seven patients had not progressed after 43 months.
Conclusions
The GAP trial demonstrated that perioperative nab-gem was tolerable. Although the primary endpoint of an 85% R0 rate was not met, the R0 rate was similar to trials using a > 1 mm R0 resection definition, and survival rates were comparable with recent adjuvant studies.


Similar content being viewed by others
References
Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022.
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74(11):2913–21.
Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310(14):1473–81.
Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection. JAMA. 2010;304(10):1073–81.
Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388(10041):248–57.
Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 2018;379:2395–406.
Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011–24.
Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. New Engl J Med. 2013;369(18):1691–703.
Tempero MA, Cardin DB, Goldstein D, et al. APACT: phase III randomized trial of adjuvant treatment with nab-paclitaxel (nab-P) plus gemcitabine (Gem) versus Gem alone in patients (pts) with resected pancreatic cancer (PC). J Clin Oncol. 2016;34(4 Suppl):TPS473.
Fathi A, Christians KK, George B, et al. Neoadjuvant therapy for localized pancreatic cancer: guiding principles. J Gastrointest Oncol. 2015;6(4):418–29.
Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
Hofheinz R-D, Wenz F, Post S, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13(6):579–88.
Gillen S, Schuster T, Meyer zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLOS Med. 2010;7(4):e1000267.
Chang DK, Johns AL, Merrett ND, et al. Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol. 2009;27(17):2855–62.
Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(21):3496–502.
Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(21):3487–95.
Jang JY, Han Y, Lee H, et al. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open-label, multicenter phase 2/3 trial. Ann Surg. 2018;268(2):215–22.
Van Tienhoven G, Versteijne E, Suker M, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC-1): a randomized, controlled, multicenter phase III trial [abstract no. LBA4002]. J Clin Oncol. 2018. https://doi.org/10.1200/JCO.2018.36.18_suppl.LBA4002.
Palmer D, Stocken D, Hewitt H, et al. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol. 2007;14(7):2088–96.
Heinrich S, Pestalozzi B, Schafer M, et al. Prospective phase II trial of neoadjuvant chemotherapy with gemcitabine and cisplatin for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(15):2526–31.
O’Reilly E, Perelshteyn A, Jarnagin W, et al. A single-arm, nonrandomized phase II trial of neoadjuvant gemcitabine and oxaliplatin in patients with resectable pancreas adenocarcinoma. Ann Surg. 2014;260(1):142–8.
Lutfi W, Talamonti MS, Kantor O, et al. Perioperative chemotherapy is associated with a survival advantage in early stage adenocarcinoma of the pancreatic head. Surgery. 2016;160(3):714–24.
Mokdad AA, Minter RM, Zhu H, et al. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: a propensity score matched analysis. J Clin Oncol. 2017;35(5):515–22.
National Comprehensive Cancer Network. NCCN guidelines for patients: pancreatic cancer. 2017. https://www.nccn.org/patients/guidelines/pancreatic/files/assets/basic-html/page-1.html.
Al-Hawary MM, Francis IR, Chari ST, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology. 2014;270(1):248–60.
Ryan R, Gibbons D, Hyland JM, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47(2):141–6.
Chuong MD, Frakes JM, Figura N, et al. Histopathologic tumor response after induction chemotherapy and stereotactic body radiation therapy for borderline resectable pancreatic cancer. J Gastrointest Oncol. 2015;7(2):221–7.
Vickers MM, Lee C, Tu D, et al. Significance of baseline and change in quality of life scores in predicting clinical outcomes in an international phase III trial of advanced pancreatic cancer: NCIC CTG PA.3. Pancreatology. 2016;16(6):1106–12.
Tajima H, Ohta T, Kitagawa H, et al. Pilot study of neoadjuvant chemotherapy with gemcitabine and oral S-1 for resectable pancreatic cancer. Exp Ther Med. 2012;3(5):787–92.
Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246(1):52–60.
Hartwig W, Hackert T, Hinz U, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg. 2011;254(2):311–9.
Royal College of Pathologists of Australasia. Cancer of the exocrine pancreas, ampulla of Vater and distal common bile duct. Structured reporting protocol. 1st edition. Sydney: Royal College of Pathologists of Australasia; 2014.
Shinoto M, Yamada S, Yasuda S, et al. Phase 1 trial of preoperative, short-course carbon-ion radiotherapy for patients with resectable pancreatic cancer. Cancer. 2013;119(1):45–51.
Turrini O, Ychou M, Moureau-Zabotto L, et al. Neoadjuvant docetaxel-based chemoradiation for resectable adenocarcinoma of the pancreas: new neoadjuvant regimen was safe and provided an interesting pathologic response. Eur J Surg Oncol. 2010;36(10):987–92.
Alvarez-Gallego R, Cubillo A, Garralda E, et al. Pathological response to neoadjuvant gemcitabine plus nabpaclitaxel in pancreatic adenocarcinoma to improve survival. J Clin Oncol. 2016;34(15 Suppl):4109.
Ielpo B, Duran H, Diaz E, et al. Preoperative treatment with gemcitabine plus nab-paclitaxel is a safe and effective chemotherapy for pancreatic adenocarcinoma. Eur J Surg Oncol. 2016;42(9):1394–400.
MacKenzie S, Zen H, McCahill LE, et al. A pilot phase II multicenter study of nab-paclitaxel (Nab-P) and gemcitabine (G) as preoperative therapy for potentially resectable pancreatic cancer (PC). J Clin Oncol. 2013;31(15Suppl):4038.
Sliesoriatis S, Desai NV, Trevino JG, et al. Final results for gemcitabine with nab-paclitaxel in neoadjuvant treatment of resectable pancreatic adenocarcinoma: GAIN-1 study. J Clin Oncol. 2014;32(15 Suppl):e15201.
Chandrasegaram MD, Goldstein D, Simes J, et al. Meta-analysis of radical resection rates and margin assessment in pancreatic cancer. Br J Surg. 2015;102(12):1459–72.
Konstantinidis IT, Warshaw AL, Allen JN, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a ‘true’ R0 resection? Ann Surg. 2013;257(4):731–6.
Mathur A, Ross SB, Luberice K, et al. Margin status impacts survival after pancreaticoduodenectomy but negative margins should not be pursued. Am Surg. 2014;80(4):353–60.
Verbeke C, Lohr M, Karlsson JS, Del Chiaro M. Pathology reporting of pancreatic cancer following neoadjuvant therapy: challenges and uncertainties. Cancer Treat Rev. 2015;41(1):17–26.
Sohal D, McDonough S, Ahmad SA, et al. SWOG S1505: Initial findings on eligibility and neoadjuvant chemotherapy experience with mfolfirinox versus gemcitabine/nab-paclitaxel for resectable pancreatic adenocarcinoma. J Clin Oncol. 2019;37(4 Suppl):414.
Mornex F, Girard N, Scoazec JY, et al. Feasibility of preoperative combined radiation therapy and chemotherapy with 5-fluorouracil and cisplatin in potentially resectable pancreatic adenocarcinoma: the French SFRO-FFCD 97-04 phase II trial. Int J Radiat Oncol Biol Phys. 2006;65(5):1471–8.
Scheithauer W, Ramanathan RK, Moore M, et al. Dose modification and efficacy of nab-paclitaxel plus gemcitabine vs. gemcitabine for patients with metastatic pancreatic cancer: phase III MPACT trial. J Gastrointest Oncol. 2016;7(3):469–78.
Serrano PE, Herman JM, Griffith KA, et al. Quality of life in a prospective multi-center phase II trial of neoadjuvant full dose gemcitabine, oxaliplatin and radiation in patients with resectable or borderline resectable pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2014;90(2):270–7.
Liao WC, Chien KL, Lin YL, et al. Adjuvant treatments for resected pancreatic adenocarcinoma: a systematic review and network meta-analysis. Lancet Oncol. 2013;14(11):1095–103.
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25.
Tempero MA, Reni M, Riess H, et al. Randomized trial of adjuvant nab-paclitaxel plus gemcitabine (nab-P/G) vs gemcitabine (G) for surgically resected pancreatic adenocarcinoma. J Clin Oncol. 2019;37(15 Suppl):4000.
Versteijne E, Vogel JA, Besselink MG, et al. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg. 2018;105(8):946–58.
Ettrich TJ, Berger AW, Muche R, et al. NEONAX: Neoadjuvant plus adjuvant or only adjuvant nab-paclitaxel plus gemcitabine for resectable pancreatic cancer: a phase II study of the AIO Pancreatic Cancer Group. J Clin Oncol. 2014;32(12 Suppl):tps4158.
GAP Investigators
Trial Management Committee: A. Barbour (chair), D. Goldstein, J.S. Samra, M. Harris, Y.J. Chua, M. Burge, N. O’Rourke, S. Yip, V. Gebski, J. Simes, R. Hicks.
Independent Data Safety Monitoring Committee: A. Coates (chair), H. Gurney, V. Do, I. Marschner.
Clinical Trials Centre: J. Mitchell, M. Donoghoe, V. Gebski, J. Simes, S. Goldstone, S. Yip, J. Simes.
Participating Medical Oncologists: Y.J. Chua (The Canberra Hospital, Canberra, ACT, Australia), N. Pavlakis (Royal North Shore Hospital, Sydney, NSW, Australia), D. Goldstein (Prince of Wales Hospital, Sydney, NSW, Australia), M. Aghmesheh (Southern Medical Day Care Centre, Woolongong, NSW, Australia), M. Burge (Royal Brisbane and Women’s Hospital, Brisbane, QLD, Australia), W. Joubert (Icon Cancer Care Wesley, Brisbane, QLD, Australia), D. Grimes (Greenslopes Private Hospital, Brisbane, QLD, Australia), M. Harris (Monash Medical Centre, Melbourne, VIC, Australia).
Participating Surgeons: J.S. Samra, K.S. Haghighi, P. Truskett, S. Bagia, M. Jaber (Sydney, NSW, Australia); S. Gananadha (Canberra, ACT, Australia); N. O’Rourke, R. Bryant, L. Nathanson, D. Cavallucci, A. Barbour, J. Fawcett, T. O’Rourke (Brisbane, QLD, Australia); D. Croagh, R. Jones (Melbourne, VIC, Australia).
Funding
Specialized Therapeutics Australia provided funding support and supplied nab‐paclitaxel; gemcitabine was available on the Pharmaceutical Benefits Scheme (PBS). This study was partly funded by the GAP-T NHMRC Project Grant (APP1026563). Trial management and statistical support was undertaken by the NHMRC-CTC. The AGITG was the legal sponsor and the trial was registered with the Australian and New Zealand Clinical Trial Registry (ACTRN12611000848909). The GAP study was conducted under the auspices of the AGITG, the legal sponsor, independently of the funders. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Consortia
Contributions
AB and DG had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study Concept and Design: Barbour, Gebski, Kench, Goldstein. Acquisition, Analysis, or Interpretation of Data: All authors. Drafting of the Manuscript: Barbour, Goldstein, Donoghoe, Gebski, Chan. Critical Revision of the Manuscript for Important Intellectual Content: All authors. Statistical Analysis: Donoghoe, Gebski. Obtained Funding: Barbour, Goldstein, Kench. Administrative, Technical, or Material Support: Barbour, Goldstein, Mitchell, Chan. Study Supervision: Barbour, Goldstein, Mitchell, Chan, Chua, Samra, Burge, Harris, O’Rourke, Kench. Additional Contributions: John Simes from the CTC provided input into the study concept and design, critical revision of the manuscript, and statistical advice. Jonathon Fawcett from the Queensland Liver Transplant Service, Brisbane, QLD, Australia, provided independent assessment of pathology reports. Sherilyn Goldstone from the CTC assisted with manuscript preparation, and Chris Brown from the CTC is acknowledged for statistical work. Manju Chandrasegaram, AGITG Research Fellow and Hepatopancreatobiliary (HPB) and General Surgeon reviewed the surgical literature to inform this paper. These people were not specifically compensated for their work. Local investigators, surgeons, and data managers gathered the data, which were submitted to the CTC in Sydney, NSW, Australia, via an electronic data collection system.
Corresponding author
Ethics declarations
Disclosure
None of the authors has declared any potential conflicts of interest with respect to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barbour, A.P., Samra, J.S., Haghighi, K.S. et al. The AGITG GAP Study: A Phase II Study of Perioperative Gemcitabine and Nab-Paclitaxel for Resectable Pancreas Cancer. Ann Surg Oncol 27, 2506–2515 (2020). https://doi.org/10.1245/s10434-020-08205-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-08205-2