Abstract
Background
The standard of care for breast cancer patients treated with neoadjuvant chemotherapy (NAC) who have a positive sentinel lymph node (+SLN) after NAC is completion axillary lymph node dissection (ALND). This study aimed to develop a nomogram to predict additional nodal disease in patients with +SLN after NAC.
Methods
The study reviewed patients 18 years of age or older who had invasive breast cancer treated with NAC followed by SLN surgery with +SLN and ALND between 2006 and 2017 at the authors’ institution. Factors predictive of positive non-SLNs were analyzed using uni- and multivariable logistic regression.
Results
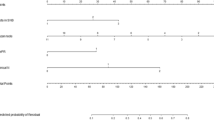
The study identified 120 patients with +SLN after NAC and ALND. Of these patients, 30.8% were clinically node-negative (cN−), and 69.2% were clinically node-positive (cN+) before NAC. Tumor biology was human epidermal growth factor receptor 2-positive (HER2+) for 20%, hormone receptor-positive (HR+)/HER2− for 66.7%, and triple-negative breast cancer (TNBC) for 13.3% of the patients. Additional nodal disease was found on ALND for 63.3% of the patients. In the univariate analysis, the factors predictive of positive non-SLNs were biologic subtype (TNBC and HR+/HER2− vs HER2+; p < 0.001), higher grade (p = 0.047), higher pT category (p = 0.02), SLN extranodal extension (p = 0.03), larger SLN metastasis size (p < 0.001), and higher number of +SLNs (p = 0.02). The factors significant in the multivariable analysis included number of +SLNs, grade 3 vs grade 1 or 2, HER2+ versus HER2−, cN+ versus cN−, and larger SLN metastasis size. The resulting model showed excellent discrimination (area under the curve, 0.82; 95% confidence interval, 0.74–0.90) and good calibration (p = 0.54, Hosmer–Lemeshow).
Conclusion
A clinical prediction model incorporating biologic subtype, grade, clinical node status, size of the largest SLN metastasis, and number of +SLNs can help physicians and patients estimate the likelihood of additional nodal disease and may be useful for guiding decision making regarding axillary management.


Similar content being viewed by others
References
Van Zee KJ, Manasseh DM, Bevilacqua JL, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10(10):1140–51.
Lambert LA, Ayers GD, Hwang RF, et al. Validation of a breast cancer nomogram for predicting nonsentinel lymph node metastases after a positive sentinel node biopsy. Ann Surg Oncol. 2006;13(3):310–20.
Mittendorf EA, Hunt KK, Boughey JC, et al. Incorporation of sentinel lymph node metastasis size into a nomogram predicting nonsentinel lymph node involvement in breast cancer patients with a positive sentinel lymph node. Ann Surg. 2012;255(1):109–15.
Memorial Sloan Kettering Cancer Center Breast Cancer Nomogram for Additional Non-SLN Metastases. Retrieved 2 April 2018 at http://nomograms.mskcc.org/breast/BreastAdditionalNonSLNMetastasesPage.aspx.
MD Anderson Cancer Center Breast Cancer Nomogram to Predict Additional Positive Non-SLN, without Neoadjuvant Chemotherapy. Retrieved 2 April 2018 at http://www3.mdanderson.org/app/medcalc/bc_nomogram2/index.cfm?pagename=nsln.
Heinze G, Puhr R. Bias-reduced and separation-proof conditional logistic regression with small or sparse data sets. Stat Med. 2010;29(7–8):770–7.
Furnival GM, Wilson RW. Regressions by leaps and bounds. J Technometrics. 1974;16(4):499–511.
Harrell, FE. Regression Modeling Strategies (2015). R package version 4.3-0. Retrieved 1 November 2017 at http://CRAN.R-project.org/package=rms.
Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer. The ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455–61.
Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–18.
Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258–64.
Boughey JC, Suman VJ, Mittendorf EA, et al. Factors affecting sentinel lymph node identification rate after neoadjuvant chemotherapy for breast cancer patients enrolled in ACOSOG Z1071 (Alliance). Ann Surg. 2015;261(3):547–52.
Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0-T4, N1-2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (Alliance). Ann Surg. 2016;263(4):802–7.
Caudle AS, Yang WT, Krishnamurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34(10):1072–8.
Comparison of Axillary Lymph Node Dissection With Axillary Radiation for Patients With Node-Positive Breast Cancer Treated With Chemotherapy. Retrieved 25 June 2018 at https://clinicaltrials.gov/ct2/show/NCT01901094.
Jeruss JS, Newman LA, Ayers GD, et al. Factors predicting additional disease in the axilla in patients with positive sentinel lymph nodes after neoadjuvant chemotherapy. Cancer. 2008;112(12):2646–54.
Ryu JM, Lee SK, Kim JY, et al. Predictive factors for nonsentinel lymph node metastasis in patients with positive sentinel lymph nodes after neoadjuvant chemotherapy: nomogram for predicting nonsentinel lymph node metastasis. Clin Breast Cancer. 2017;17(7):550–8.
Gimbergues P, Abrial C, Durando X, et al. Clinicopathological factors and nomograms predicting nonsentinel lymph node metastases after neoadjuvant chemotherapy in breast cancer patients. Ann Surg Oncol. 2009;16(7):1946–51.
Hwang EF, Krishnamurthy S, Hunt KK, et al. Clinicopathologic factors predicting involvement of nonsentinel axillary nodes in women with breast cancer. Ann Surg Oncol. 2003;10(3):248–54.
Barranger E, Coutant C, Flahault A, Delpech Y, Darai E, Uzan S. An axilla scoring system to predict nonsentinel lymph node status in breast cancer patients with sentinel lymph node involvement. Breast Cancer Res Treat. 2005;91(2):113–9.
Boughey JC, McCall LM, Ballman KV, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) prospective multicenter clinical trial. Ann Surg. 2014;260(4):608–14; (discussion 614–6).
Kantor O, Sipsy LM, Yao K, James TA. A predictive model for axillary node pathologic complete response after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol. 2018;25(5):1304–11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors have no conflicts of interest to report.
Rights and permissions
About this article
Cite this article
Barron, A.U., Hoskin, T.L. & Boughey, J.C. Predicting Non-sentinel Lymph Node Metastases in Patients with a Positive Sentinel Lymph Node After Neoadjuvant Chemotherapy. Ann Surg Oncol 25, 2867–2874 (2018). https://doi.org/10.1245/s10434-018-6578-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-6578-3