Abstract
Background
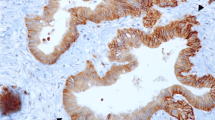
This study aimed to examine the prognostic relevance of glucose transporter type 1 (GLUT-1), which is a key regulator of the glucose metabolism. In particular, the study aimed to examine the association between GLUT-1 expression and the therapeutic effect of chemoradiotherapy (CRT) in pancreatic ductal adenocarcinoma (PDAC).
Methods
Patients with PDAC were enrolled in the study. Patients with distant metastases and those who received only chemotherapy as treatment were excluded from the study. Specimens for immunohistochemical evaluations were obtained through surgical resection and endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) of the primary tumor before any treatment.
Results
This study included 197 patients. Of these 197 patients, 100 underwent upfront surgery, and 97 received neoadjuvant CRT (NACRT), which was performed mainly for patients with locally advanced tumors. Of the 97 patients who received NACRT, 21 later underwent surgical resection. For the patients who underwent upfront surgery, low GLUT-1 expression was an independent factor for a better prognosis. For the patients who underwent NACRT, low GLUT-1 expression was significantly associated with greater tumor size reduction, a higher resection rate, and a better prognosis. Additionally, GLUT-1 expression was significantly increased after NACRT treatment.
Conclusions
Among the patients with PDAC, those with low GLUT-1 expression in the primary tumor had a better prognosis those with high GLUT-1 expression. Moreover, the patients with low GLUT-1 expression displayed a better therapeutic response to NACRT.



Similar content being viewed by others
References
Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267.
Ying JE, Zhu LM, Liu BX. Developments in metastatic pancreatic cancer: is gemcitabine still the standard? World J Gastroenterol. 2012;18:736–45.
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29.
Small W Jr, Berlin J, Freedman GM, et al. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: a multicenter phase II trial. J Clin Oncol. 2008; 26:942–47.
Shinchi H, Maemura K, Mataki Y, et al. A phase II study of oral S-1 with concurrent radiotherapy followed by chemotherapy with S-1 alone for locally advanced pancreatic cancer. J Hepatobiliary Pancreat Sci. 2012;19:152–58.
Takahashi H, Akita H, Tomokuni A, et al. Preoperative gemcitabine-based chemoradiation therapy for borderline resectable pancreatic cancer: impact of venous and arterial involvement status on surgical outcome and pattern of recurrence. Ann Surg. 2016;264:1091–97.
Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330:1340–44.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Warburg O. On the origin of cancer cells. Science. 1956;123:309–14.
Meijer TW, Kaanders JH, Span PN, Bussink J. Targeting hypoxia, HIF-1, and tumor glucose metabolism to improve radiotherapy efficacy. Clin Cancer Res. 2012;18:5585–94.
Luo XM, Xu B, Zhou ML, Bao YY, Zhou SH, Fan J, Lu ZJ. Co-inhibition of GLUT-1 expression and the PI3K/Akt signaling pathway to enhance the radiosensitivity of laryngeal carcinoma xenografts in vivo. PloS One. 2015;10:e0143306.
Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654–62.
Zhang C, Liu J, Liang Y, et al. Tumour-associated mutant p53 drives the Warburg effect. Nat Commun. 2013;4:2935.
Vaupel P, Mayer A, Hockel M. Tumor hypoxia and malignant progression. Methods Enzymol. 2004;381:335–54.
Shin SH, Kim SC, Hong SM, Kim YH, Song KB, Park KM, Lee YJ. Genetic alterations of K-ras, p53, c-erbB-2, and DPC4 in pancreatic ductal adenocarcinoma and their correlation with patient survival. Pancreas. 2013;42:216–22.
Kim H, Saka B, Knight S, et al. Having pancreatic cancer with tumoral loss of ATM and normal TP53 protein expression is associated with a poorer prognosis. Clin Cancer Res, 2014;20:1865–72.
Younes M, Brown RW, Mody DR, Fernandez L, Laucirica R. GLUT1 expression in human breast carcinoma: correlation with known prognostic markers. Anticancer Res. 1995;15:2895–8.
Haber RS, Rathan A, Weiser KR, et al. GLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosis. Cancer. 1998;83:34–40.
Kawamura T, Kusakabe T, Sugino T, et al. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer. 2001;92:634–41.
Melstrom LG, Salabat MR, Ding XZ, et al. Apigenin down-regulates the hypoxia response genes: HIF-1alpha, GLUT-1, and VEGF in human pancreatic cancer cells. J Surg Res. 2011;167:173–81.
Basturk O, Singh R, Kaygusuz E, Kaygusuz E, Balci S, Dursun N, Adsay NV. GLUT-1 expression in pancreatic neoplasia: implications in pathogenesis, diagnosis, and prognosis. Pancreas. 2011;40:187–92.
Sharen G, Peng Y, Cheng H, Liu Y, Shi Y, Zhao J. Prognostic value of GLUT-1 expression in pancreatic cancer: results from 538 patients. Oncotarget. 2017;8:19760–7.
Sobin LH, Gospodarowicz MK, Wittekind C, International Union against Cancer (2010) TNM Classification of Malignant Tumours. 7th ed.Wiley-Blackwell, Chichester.
Maemura K, Shinchi H, Noma H, et al. Comparison of hyper-fractionated accelerated and standard fractionated radiotherapy with concomitant low-dose gemcitabine for unresectable pancreatic cancer. Anticancer Res. 2008;28:2369–72.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Chikamoto A, Inoue R, Komohara Y, et al. Preoperative high maximum standardized uptake value in association with glucose transporter 1 predicts poor prognosis in pancreatic cancer. Ann Surg Concol. 2017;24:2040–6.
Erickson RA. EUS-guided FNA. Gastrointest Endosc. 2004;60:267–79.
Higashi M, Yokoyama S, Yamamoto T, et al. Mucin expression in endoscopic ultrasound-guided fine-needle aspiration specimens is a useful prognostic factor in pancreatic ductal adenocarcinoma. Pancreas. 2015;44:728–34.
Kurahara H, Maemura K, Mataki Y, Sakoda M, Shinchi H, Natsugoe S. Impact of p53 and PDGFR-beta expression on metastasis and prognosis of patients with pancreatic cancer. World J Surg. 2016;40:1977–84.
Yamada R, Mizuno S, Uchida K, et al. Human equilibrative nucleoside transporter 1 expression in endoscopic ultrasonography-guided fine-needle aspiration biopsy samples is a strong predictor of clinical response and survival in the patients with pancreatic ductal adenocarcinoma undergoing gemcitabine-based chemoradiotherapy. Pancreas. 2016;45:761–71.
Acknowledgment
This study was funded by Grants-in-Aid for Scientific Research (26462067) from the Japan Society for the Promotion of Science, Ministry of Health, Labour and Welfare, Japan.
Disclosure
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2018_6357_MOESM5_ESM.jpg
Fig. S1 Kaplan–Meier survival curves from the initial treatment (surgery) for patients who underwent upfront surgery.. Supplementary material 5 (JPEG 690 kb)
10434_2018_6357_MOESM6_ESM.jpg
Fig. S2 Kaplan–Meier survival curves from the initial treatment for patients who underwent neoadjuvant chemoradiotherapy (NACRT). a Overall survival (OS) of patients who underwent surgical resection after NACRT. b Recurrence-free survival of patients who underwent surgical resection after NACRT. c OS of patients who did not undergo surgical resection after NACRT. d Progression-free survival of patients who did not undergo surgical resection after NACRT. Supplementary material 6 (JPEG 1027 kb)
10434_2018_6357_MOESM7_ESM.jpg
Fig. S3 Kaplan–Meier survival curves from the initial treatment for patients who underwent neoadjuvant chemoradiotherapy (NACRT). Eight patients experienced a low-to-high change in glucose transporter type 1 (GLUT-1) expression due to NACRT. Supplementary material 7 (JPEG 712 kb)
Rights and permissions
About this article
Cite this article
Kurahara, H., Maemura, K., Mataki, Y. et al. Significance of Glucose Transporter Type 1 (GLUT-1) Expression in the Therapeutic Strategy for Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol 25, 1432–1439 (2018). https://doi.org/10.1245/s10434-018-6357-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-6357-1