Abstract
Background
Recent trials have suggested the feasibility of performing a sentinel lymph node biopsy (SNB) following neoadjuvant chemotherapy (NAC). The selection of suitable patients for this approach remains controversial. We developed a predictive model to identify patients most likely to benefit from SNB following NAC.
Methods
The National Cancer Data Base was used to identify patients with clinically node positive (cN+) breast cancer undergoing NAC followed by breast surgery and axillary lymph node dissection (ALND). Patients were randomly assigned to a 70% testing or 30% validation cohort for model development. A predictive model was built based on significant factors associated with pathologic nodal response (pN0) and breast response.
Results
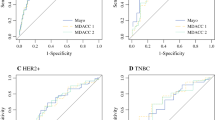
Using the testing cohort (n = 13,396), multivariate regression was used to identify predictors of pN0 based on preoperative factors. Younger age, hormone receptor (HR)-negative/Her2-negative, HR-positive/Her2-positive, HR-negative/Her2-positive, high-grade, ductal histology, cN1 versus cN2, and extent of breast response were all significant independent predictors of pN0 on adjusted analysis. The odds ratios translated into a 10-point scale correlating to a stepwise increase in pN0 response. The area under the curve for the ROC curves for the testing and validation cohorts was 0.781 and 0.788, respectively (p < 0.01).
Conclusions
Our model incorporates known preoperative factors to predict the likelihood of pN0 response in patients with cN+ disease who undergo NAC. For patients with high scores, SNB should be considered over ALND, because these patients have a greater likelihood of having negative nodes at final pathology.

Similar content being viewed by others
References
Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. Alliance for Clinical Trials in Oncology. JAMA. 2013;310(14):1455–61. https://doi.org/10.1001/jama.2013.278932.
Mautner SK, Cody HS 3rd. Sentinel node biopsy after neoadjuvant chemotherapy for node-positive breast cancer: does axillary ultrasound improve performance? J Clin Oncol. 2015;33(30):3375–8. https://doi.org/10.1200/jco.2014.60.3316.
Morrow M, Dang CT. Sentinel node biopsy after neoadjuvant chemotherapy: a new standard for patients with axillary metastases? JAMA. 2013;310(14):1455–61.
Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–18. https://doi.org/10.1016/s1470-2045(13)70166-9.
Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–85.
van Nijnatten TJ, et al. Prognosis of residual axillary disease after neoadjuvant chemotherapy in clinically node-positive breast cancer patients: isolated tumor cells and micrometastases carry a better prognosis than macrometastases. Breast Cancer Res Treat. 2017 Feb 17. https://doi.org/10.1007/s10549-017-4157-0.
Mougalian SS, et al. Ten-year outcomes of patients with breast cancer with cytologically confirmed axillary lymph node metastases and pathologic complete response after primary systemic chemotherapy. JAMA Oncol. 2016;2(4):508–16. https://doi.org/10.1001/jamaoncol.2015.4935.
Kim MM, et al. Pathologic complete response to neoadjuvant chemotherapy with trastuzumab predicts for improved survival in women with HER2-overexpressing breast cancer. Ann Oncol. 2013;24(8):1999–2004. https://doi.org/10.1093/annonc/mdt131.
Broglio KR, et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol. 2016;2(6):751–60. https://doi.org/10.1001/jamaoncol.2015.6113.
Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–900.
Peintinger F, Kuerer HM, Anderson K, et al. Accuracy of the combination of mammography and sonography in predicting tumor response in breast cancer patients after neoadjuvant chemotherapy. Ann Surg Oncol. 2006;13(11):1443–9.
Marinovich ML, Houssami N, Macaskill P, et al. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J Natl Cancer Inst. 2013;105(5):321–33.
Kim JY, Park HS, Kim S, et al. Prognostic nomogram for prediction of axillary pathologic complete response after neoadjuvant chemotherapy in cytologically proven node-positive breast cancer. Medicine. 2015;94(43):e1720.
Schipper RJ, Moossdorff M, Nelemans PJ, et al. A model to predict pathologic complete response of axillary lymph nodes to neoadjuvant chemo(immuno)therapy in patients with clinically node-positive breast cancer, clinical breast cancer, 2014;14:315–22, ISSN 1526-8209, http://dx.doi.org/10.1016/j.clbc.2013.12.015.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kantor, O., Sipsy, L.M., Yao, K. et al. A Predictive Model for Axillary Node Pathologic Complete Response after Neoadjuvant Chemotherapy for Breast Cancer. Ann Surg Oncol 25, 1304–1311 (2018). https://doi.org/10.1245/s10434-018-6345-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-6345-5