Abstract
Background
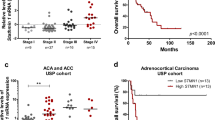
Adrenocortical carcinoma (ACC) is a rare endocrine malignancy with a poor prognosis and few therapeutic options. Stathmin1 (STMN1) is a cytosolic protein involved in microtubule dynamics through inhibition of tubulin polymerization and promotion of microtubule depolymerization, which has been implicated in carcinogenesis and aggressive behavior in multiple epithelial malignancies. We aimed to evaluate expression of STMN1 in ACC and to elucidate how this may contribute to its malignant phenotype.
Methods
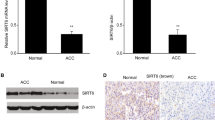
STMN1 was identified by RNA sequencing as a highly differentially expressed gene in human ACC samples compared with benign adrenal tumors. Expression was confirmed by quantitative reverse transcription-polymerase chain reaction (qRT-PCR), Western blot, and immunohistochemical (IHC) staining of a tissue microarray (TMA) from two independent cohorts. The biologic relevance of STMN1 was investigated in NCI-H295R cells by lentivirus-mediated silencing.
Results
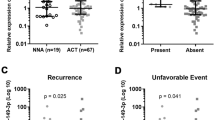
Differential gene expression demonstrated an eightfold increase in STMN1 messenger RNA (mRNA) in malignant compared with benign adrenal tissue. IHC showed significantly higher expression of STMN1 protein in ACC compared with normal and benign tissues. STMN1 knockdown in an ACC cell line resulted in decreased cell viability, cell-cycle arrest at G0/G1, and increased apoptosis in serum-starved conditions compared with scramble short hairpin RNA (shRNA) controls. STMN1 knockdown also decreased migration, invasion, and anchorage-independent growth compared with controls.
Conclusions
STMN1 is overexpressed in human ACC samples, and knockdown of this target in vitro resulted in a less aggressive phenotype of ACC, particularly under serum-starved conditions. Further study is needed to investigate the feasibility of interfering with STMN1 as a potential therapeutic target.





Similar content being viewed by others
References
Fassnacht M, Libe R, Kroiss M, Allolio B. Adrenocortical Carcinoma: A Clinician’s Update. Nat Rev Endocrinol. 2011;7:323–35.
Fassnacht M, Kroiss M, Allolio B. Update in adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98(12):4551–64.
Icard P, Goudet P, Charpenay C, Andreassian B, Carnaille B, Chapuis Y, et al. Adrenocortical carcinomas: Surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons Study Group. World J Surg. 2001;25(7):891–7.
Bilimoria KY, Shen WT, Elaraj D, Bentrem DJ, Winchester DJ, Kebebew E, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113(11):3130–6.
Datta J, Roses RE. Surgical Management of Adrenocortical Carcinoma: An Evidence-Based Approach. Surg Oncol Clin N Am. 2016;25(1):153–70.
Libé R. Adrenocortical carcinoma (ACC): diagnosis, prognosis, and treatment. Front Cell Dev Biol. 2015;3:45.
Creemers SG, Hofland L, Korpershoek E, Franssen GJ., van Kemenade FJ, de Herder WW, et al. Future directions in the diagnosis and medical treatment of adrenocortical carcinoma. Endocr Relat Cancer. 2016;23(1):R43-69.
Wortmann S, Quinkler M, Ritter C, Kroiss M, Johanssen S, Hahner S, et al. Bevacizumab plus capecitabine as a salvage therapy in advanced adrenocortical carcinoma. Eur J Endocrinol. 2010;162:349–56.
O’Sullivan C, Edgerly M, Velarde M, Wilkerson J, Venkatesan AM, Pittaluga S, et al. The VEGF inhibitor axitinib has limited effectiveness as a therapy for adrenocortical cancer. J Clin Endocrinol Metab. 2014;99(4):1291–7.
Fassnacht M, Berruti A, Baudin E, Demeure MJ, Gilbert J, Haak H, et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol. 2015;16(4):426–35.
Costa R, Carneiro BA, Tavora F, Pai SG, Kaplan B, Chae YK, et al. The challenge of developmental therapeutics for adrenocortical carcinoma. Oncotarget. 2016;7(29):46734–49.
Berruti A, Sperone P, Ferrero A, Germano A, Ardito A, Priola M, et al. Phase II study of weekly paclitaxel and sorafenib as second/third-line therapy in patients with adrenocortical carcinoma. Eur J Endocrinol. 2012;166:451–8.
Rubin CI, Atweh GF. The role of stathmin in the regulation of the cell cycle. J Cell Biochem. 2004;93(2):242–50.
Akhtar J, Wang Z, Yu C, Zhang ZP, Bi MM. STMN-1 Gene: A Predictor of Survival in Stage IIA Esophageal Squamous Cell Carcinoma After Ivor-Lewis Esophagectomy. Ann Surg Oncol. 2014;21(1):315–21.
He X, Liao Y, Lu W, Xu G, Tong H, Ke J, et al. Elevated STMN1 promotes tumor growth and invasion in endometrial carcinoma. Tumour Biol. 2016;37(7):9951–8.
Hsieh S, Huang S, Yu M, Yeh T, Chen T, Lin Y, et al. Stathmin1 overexpression associated with polyploidy, tumor-cell invasion, early recurrence, and poor prognosis in human hepatoma. Mol Carcinog. 2010;49(5):476–87.
Kang W, Tong JHM, Chan AWH, Lung RWM, Chau SL, Wong QWL, et al. Stathmin1 Plays Oncogenic Role and Is a Target of MicroRNA-223 in Gastric Cancer. PLOS One. 2012;7(3):33919.
Kouzu Y, Uzawa K, Koike H, Saito K, Nakashima D, Higo M, et al. Overexpression of stathmin in oral squamous-cell carcinoma: correlation with tumour progression and poor prognosis. Br J Cancer. 2006;94:717–23.
Kuang X, Chen L, Zhang Z, Liu Y, Zheng Y. Stathmin and phospho-stathmin protein signature is associated with survival outcomes of breast cancer patients. Oncotarget. 2015;6(26):22227–38.
Zhang H, Guo X, Guo S, Wang Q, Chen X. STMN1 in colon cancer: expression and prognosis in Chinese patients. Eur Rev Med Pharmacol Sci. 2016;20:2038–44.
Yu W, Tan XF, Tan HT, Lim TK, Chung MCM. Unbiased Proteomic and Transcript Analyses Reveal that Stathmin-1 Silencing Inhibits Colorectal Cancer Metastasis and Sensitizes to 5-Fluorouracil Treatment. Mol Cancer Res. 2014;12(12):1717–28.
Byme F, Yang L, Philliips P, Hansford L, Fletcher J, Ormandy C, et al. RNAi-mediated stathmin suppression reduces lung metastasis in an orthotopic neuroblastoma mouse model. Oncogene. 2014;33(7):882–90.
Wang S, Akhtar J, Wang Z. Anti-STMN1 therapy improves sensitivity to antimicrotubule drugs in esophageal squamous cell carcinoma. Tumour Biol. 2015;36(10):7797–806.
Gadzar A, Oie H, Shackleton C, Chen T, Triche T, Myers C, et al. Establishment and characterization of a human adrenocortical carcinoma cell line that expresses multiple pathways of steroid biosynthesis. Cancer Res. 1990;50(17):5488–96.
Roos G, Brattsand G, Landberg G, Marklund U, Gullberg M. Expression of oncoprotein 18 in human leukemias and lymphomas. Leukemia. 1993;7(10):1538–46.
Rana S, Maples P, Senzer N, Nemunaitis J. Stathmin1: A novel therapeutic target for anticancer activity. Expert Rev Anticancer Ther. 2008;8(9):1461–70.
Akhtar J, Wang Z, Yu C, Li C-S, Shi Y-L, Liu H-J. STMN-1 is a potential marker of lymph node metastasis in distal esophageal adenocarcinomas and silencing its expression can reverse malignant phenotype of tumor cells. BMC Cancer. 2014;14:28.
Watanabe A, Suzuki H, Yokobori T, Tsukagoshi M, Altan B, Kubo N, et al. Stathmin1 regulates p27 expression, proliferation and drug resistance, resulting in poor clinical prognosis in cholangiocarcinoma. Cancer Sci. 2014;105(6):690–6.
Yuan R, Jeng Y, Chen H, Lai P, Pan H, Hsieh F, et al. Stathmin overexpression cooperates with p53 mutation and osteopontin overexpression, and is associated with tumour progression, early recurrence, and poor prognosis in hepatocellular carcinoma. J Pathol. 2006;309:549–58.
Reyes HD, Miecznikowski J, Gonzalez-Bosquet J, Devor EJ, Zhang Y, Thiel KW, et al. High stathmin expression is a marker for poor clinical outcome in endometrial cancer: an NRG oncology group/gynecologic oncology group study. Gynecol Oncol. 2017;146(2):247-253.
Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4(4):253–65.
Alli E, Bash-Babula J, Yang J-M, Hait WN. Effect of Stathmin on the Sensitivity to Antimicrotubule Drugs in Human Breast Cancer. Cancer Res. 2002;62(23):6864–9.
Alli E, Yang J, Ford J, Hait W. Reversal of stathmin-mediated resistance to paclitaxel and vinblastine in human breastcarcinoma cells. Mol Pharmacol. 2007;71(5):1233–40.
Wang Z, He R, Xia H, Wei Y, Wu S. Knockdown of STMN1 enhances osteosarcoma cell chemosensitivity through inhibition of autophagy. Oncol Lett. 2017; 13(5) 3465–70.
Zhang X, Ji JF, Yang Y, Zhang J, Shen LF. Stathmin1 increases radioresistance by enhancing autophagy in non-small-cell lung cancer cells. Onco Targets Ther. 2016;9:2565–74.
Wang Z, Jay CM, Evans, C, Kumar P, Phalon C, Rao DD, et al. Preclinical Biodistribution and Safety Evaluation of a pbi-shRNA STMN1 Lipoplex after Subcutaneous Delivery. Toxicol Sci. 2017;155(2):400–8.
Funding
This study was supported by the Weill Cornell Clinical and Translational Science Center NIH/NCATS Grant TL1TR000459.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aronova, A., Min, I.M., Crowley, M.J.P. et al. STMN1 is Overexpressed in Adrenocortical Carcinoma and Promotes a More Aggressive Phenotype In Vitro. Ann Surg Oncol 25, 792–800 (2018). https://doi.org/10.1245/s10434-017-6296-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-6296-2