Abstract
Background
A phase 1b trial was conducted to evaluate the duration of interferon-alpha (IFNα) production after intravesical administration of recombinant adenovirus-mediated interferon α2b (Ad-IFN) formulated with the excipient Syn3. The primary aim was to determine whether a second instillation 3 days after initial treatment produced prolonged urinary IFN production.
Methods
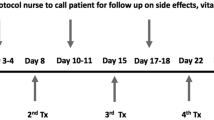
The study enrolled seven patients who experienced recurrent non-muscle invasive bladder cancer after bacillus Calmette–Guerin therapy. Each treatment consisted of intravesical instillation of SCH721015 (Syn3) and Ad-IFN at a concentration of 3 × 1011 particles/mL to a total volume of 75 mL given on days 1 and 4. The patients were followed for 12 weeks, during which the magnitude and duration of gene transfer were determined by urine INFα levels. Drug efficacy was determined by cystoscopy and biopsy, and patients who had no recurrence at 12 weeks were eligible for a second course of treatment.
Results
Seven patients were treated with an initial course (instillation on days 1 and 4). Two of the patients had a complete response at 12 weeks and received a second course of treatment. One patient remained without evidence of recurrence after a second course (total 24 weeks). One patient experienced a non-treatment-associated adverse event. Despite a transient rise in IFNα levels, sustained production was not demonstrated.
Conclusion
Previously, Ad-IFNα intravesical therapy has shown promising drug efficacy. A prior phase 1 trial with a single instillation compared similarly with the current study, suggesting that a second instillation is not necessary to achieve sufficient urinary IFNα levels.

Similar content being viewed by others
References
Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette–Guerin immunotherapy for recurrent TA, T1, and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. JURO. 2000;163:1124–9.
Yates DR, Brausi MA, Catto JWF, et al. Treatment options available for bacillus Calmette–Guérin failure in non-muscle-invasive bladder cancer. Eur Urol. 2012;62:1088–96. doi:10.1016/j.eururo.2012.08.055.
Babjuk M, Burger M, Zigeuner R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64:639–53. doi:10.1016/j.eururo.2013.06.003.
Svatek RS, Fisher MB, Matin SF, et al. Risk factor analysis in a contemporary cystectomy cohort using standardized reporting methodology and adverse event criteria. JURO. 2010;183:929–34. doi:10.1016/j.juro.2009.11.038.
Dinney CPN, Fisher MB, Navai N, et al. Phase I trial of intravesical recombinant adenovirus-mediated interferon-α2b formulated in Syn3 for BCG failures in non-muscle-invasive bladder cancer. J Urol. 2013;190(3):850–6. doi:10.1016/j.juro.2013.03.030.
Benedict WF, Tao Z, Kim C-S, et al. Intravesical Ad-IFN alpha causes marked regression of human bladder cancer growing orthotopically in nude mice and overcomes resistance to IFN-alpha protein. Mol Ther. 2004;10:525–32. doi:10.1016/j.ymthe.2004.05.027.
Sylvester RJ, van der Meijden APM, Witjes JA, Kurth K. Bacillus Calmette–Guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. JURO. 2005;174:86–91. doi:10.1097/01.ju.0000162059.64886.1c.
Dinney CPN, Greenberg RE, Steinberg GD. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette–Guérin. URO. 2013;31:1635–42. doi:10.1016/j.urolonc.2012.04.010.
Steinberg G, Bahnson R, Brosman S, Middleton R, Wajsman Z, Wehle M. Efficacy and safety of valrubicin for the treatment of bacillus Calmette–Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study Group. JURO. 2000;163:761–7. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=10687972&retmode=ref&cmd=prlinks.
Yates DR, Rouprêt M. Contemporary management of patients with high-risk non-muscle-invasive bladder cancer who fail intravesical BCG therapy. World J Urol. 2011;29:415–22. doi:10.1007/s00345-011-0681-4.
Dalbagni G. Phase II trial of intravesical gemcitabine in bacille Calmette–Guerin-refractory transitional cell carcinoma of the bladder. J Clin Oncol. 2006;24:2729–34. doi:10.1200/JCO.2005.05.2720.
Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009;55:164–76. doi:10.1016/j.eururo.2008.07.031.
Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1054 patients. J Clin Oncol. 2001;19:666–75.
Järvinen R, Kaasinen E, Sankila A, Rintala E. Long-term efficacy of maintenance bacillus Calmette–Guérin versus maintenance mitomycin C instillation therapy in frequently recurrent TaT1 tumours without carcinoma in situ: a subgroup analysis of the prospective, randomised FinnBladder I study with a 20-year follow-up. Eur Urol. 2009;56:260–5. doi:10.1016/j.eururo.2009.04.009.
Nativ O, Witjes JA, Hendricksen K, et al. Combined thermochemotherapy for recurrent bladder cancer after bacillus Calmette–Guerin. JURO. 2009;182:1313–7. doi:10.1016/j.juro.2009.06.017.
McKiernan JM. Phase I trial of intravesical docetaxel in the management of superficial bladder cancer refractory to standard intravesical therapy. J Clin Oncol. 2006;24:3075–80. doi:10.1200/JCO.2005.03.1161.
Bassi PF, Volpe A, D’Agostino D, et al. Paclitaxel-hyaluronic acid for intravesical therapy of bacillus Calmette–Guérin refractory carcinoma in situ of the bladder: results of a phase I study. JURO. 2010;185:445–9. doi:10.1016/j.juro.2010.09.073.
McKiernan JM, Barlow LJ, Laudano MA, Mann MJ, Petrylak DP, Benson MC. A phase I trial of intravesical nanoparticle albumin-bound paclitaxel in the treatment of bacillus Calmette–Guérin refractory nonmuscle invasive bladder cancer. JURO. 2011;186:448–51. doi:10.1016/j.juro.2011.03.129.
Acknowledgments
Supported in part by the National Cancer Institute of the National Institutes of Health under the following awards: The Cancer Center Support Grant P30CA016672, the MD Anderson Cancer Center SPORE in Genitourinary Cancer P50CA091846, and the Career Development Program of the MD Anderson SPORE in Genitourinary Cancer P50 CA091846. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Neema Navai and William F. Benedict contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Navai, N., Benedict, W.F., Zhang, G. et al. Phase 1b Trial to Evaluate Tissue Response to a Second Dose of Intravesical Recombinant Adenoviral Interferon α2b Formulated in Syn3 for Failures of Bacillus Calmette–Guerin (BCG) Therapy in Nonmuscle Invasive Bladder Cancer. Ann Surg Oncol 23, 4110–4114 (2016). https://doi.org/10.1245/s10434-016-5300-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5300-6