Abstract
Background
Molecular profiling in gastric cancer (GC) is important for diagnosis and treatment. In this study, we investigated signal transduction pathways that might induce chromosomal instability in GC.
Methods
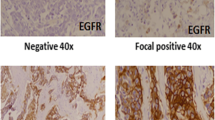
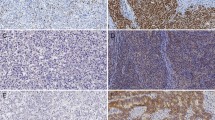
Epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), and p-AKT expression were analyzed using immunohistochemistry, and chromosomal instability was assessed by DNA aneuploidy using laser scanning cytometry, in a total of 202 GC cases.
Results
The rate of EGFR expression and p-AKT expression was 70.3 and 34.2 %, respectively, in GC patients. In total, 57.5 % of GC patients exhibited DNA aneuploidy, and p-AKT positively correlated with EGFR and HER2 (p = 0.0127 and p = 0.00031, respectively). Patients with EGFR overexpressing GC showed shorter disease-specific survival than the other cases (hazard ratio 2.00, 95 % confidence interval 1.19–3.53; p = 0.0104). Moreover, EGFR and p-AKT expression was significantly correlated with DNA aneuploidy (p = 0.0002 and p = 0.0302, respectively).
Conclusions
Our data showed that both EGFR and p-AKT overexpression were clearly associated with DNA aneuploidy. Aneuploidy could be a useful marker for therapies that target EGFR.


Similar content being viewed by others
References
Yasui W, Sentani K, Sakamoto N, Anami K, Naito Y, Oue N. Molecular Pathology of Gastric Cancer: Research and Practice. Pathol Res Pract. 2011; 207:608–12.
Cervantes A, Roselló S, Roda D, Rodríguez-Braun E. The treatment of advanced gastric cancer: current strategies and future perspectives. Ann Oncol. 2008;19:103–07.
Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9.
Wang K, Yuen ST, Xu J, et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573–82
Mertens F, Johansson B, Mitelman F. Isochromosomes in neoplasia. Genes Chro- mosomes Cancer. 1994;10:221–30.
Nam H-J, Chae S, Jang S-H, Cho H, Lee J-H. Carcinogenesis. 2010;31:1531–40
Bang YJ. Advances in the management of HER2-positive advanced gastric and gastroesophageal junction cancer. J Clin Gastroenterol. 2012;46:637–48
Ye D, Mendelsohn J, Fan Z. Augmentation of a humanized anti-HER2 mAb 4D5 induced growth inhibition by a human-mouse chimeric anti-EGF receptor mAb C225. Oncogene. 1999; 18:731–38.
Schlessinger J. Common and distinct elements in cellular signaling via EGF and FGF receptors. Science. 2004; 306:1506–07.
Kim JS, Kim MA, Kim TM, et al. Biomarker analysis in stage III–IV (M0) gastric cancer patients who received curative surgery followed by adjuvant 5-fluorouracil and cisplatin chemotherapy: epidermal growth factor receptor (EGFR) associated with favorable survival. Br J Cancer. 2009;100:732–38
Oki E, Baba H, Tokunaga E, et al. AKT phosphorylation associates with LOH of PTEN and leads to chemoresistance for gastric cancer. Int. J. Cancer. 2005:117;376–80
Radu OM, Foxwell T, Cieply K, Navina S, Dacic S, Nason KS, Davison JM. HER2 amplification in gastroesophageal adenocarcinoma: correlation of two antibodies using gastric cancer scoring criteria, H score, and digital image analysis with fluorescence in situ hybridization. Am J Clin Pathol. 2012; 137:583–94.
Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45.
Ando K, Kakeji Y, Kitao H, et al. High expression of BUBR1 is one of the factors for inducing DNA aneuploidy and progression in gastric cancer. Cancer Sci. 2010;101:639–45.
Furuya T, Uchiyama T, Murakami T et al. Relationship between chromosomal instability and intratumoral regional DNA ploidy heterogeneity in primary gastric cancers. Clin Cancer Res. 2000;6:2815–20.
Seabright, M. (1971) A rapid banding technique for human chromosomes. Lancet. 1971;30:971–72.
Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001;7:2958–70.
Galizia G, Lieto E, Ferraraccio F, et al. Prognostic significance of epidermal growth factor receptor expression in colon cancer patients undergoing curative surgery. Ann Surg Oncol. 2006;13:823–35.
Garcia I, Vizoso F, Martin A, Sanz L, Abdel-Lah O, Raigoso P, Garcia-Muniz JL. Clinical significance of the epidermal growth factor receptor and HER2 receptor in resectable gastric cancer. Ann Surg Oncol. 2003;10:234–41.
Galizia G, Lieto E, Orditura M, et al. Epidermal growth factor receptor (EGFR) expression is associated with a worse prognosis in gastric cancer patients undergoing curative surgery. World J Surg. 2007;31:1458–68.
Fusco N, Elena GR, Claudia DC. HER2 in gastric cancer: a digital image analysis in pre-neoplastic, primary and metastatic lesions. Mod Pathol. 2013; 26:1–9.
Masanori T, Kitada K. Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin Cancer Res. 2012;18:5992–6000.
Berg D, Wolff C, Malinowsky K, et al. Profiling signalling pathways in formalin-fixed and paraffin-embedded breast cancer tissues reveals cross-talk between EGFR, HER2, HER3 and uPAR. J Cell Physiol. 2012;227:204–12.
Almhanna K, Strosberg J, Malafa M. Targeting AKT protein kinase in gastric cancer. Anticancer Res. 2011;31:4387–92.
Hudis C, Swanton C, Janjigian YY, et al. A phase 1 study evaluating the combination of an allosteric AKT inhibitor (MK-2206) and trastuzumab in patients with HER2-positive solid tumors. Breast Cancer Res. 2013;19:R110. doi:10.1186/bcr3577.
Baba H, Korenaga D, Kakeji Y, Haraguchi M, Okamura T, Maehara Y. DNA ploidy and its clinical implications in gastric cancer. Surgery 2002;131:S63–70.
Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res. 2006;12:5268–72.
Zasadil LM, Britigan EM, Weaver BA. 2n or not 2n: Aneuploidy, polyploidy and chromosomal instability in primary and tumor cells. Semin Cell Dev Biol. 2013;24:370–9.
Araujo SE, Bernardo WM, Habr-Gama A, Kiss DR, Cecconello I. DNA ploidy status and prognosis in colorectal cancer: a meta-analysis of published data. Dis Colon Rectum. 2007;50:1800–10.
Habermann JK, Brucker CA, Freitag-Wolf S, et al. Genomic instability and oncogene amplifications in colorectal adenomas predict recurrence and synchronous carcinoma. Mod Pathol. 2011;24:542–55.
Ly P, Eskiocak U, Kim SB, et al. Characterization of aneuploid populations with trisomy 7 and 20 derived from diploid human colonic epithelial cells. Neoplasia. 2011;13:348–357.
Ly P, Kim SB, Kaisani AA, Marian G, Wright WE, Shay JW. Aneuploid human colonic epithelial cells are sensitive to AICAR-induced growth inhibition through EGFR degradation. Oncogene. 2013;32:3139–46.
Li L, Dutra A, Pak E, Labrie JE 3rd, et al. EGFRvIII expression and PTEN loss synergistically induce chromosomal instability and glial tumors. Neuro Oncol. 2009;11:9–21.
Mardin BR, Isokane M, Cosenza MR, Krämer A, Ellenberg J, Fry AM, Schiebel E. EGF-induced centrosome separation promotes mitotic progression and cell survival. Dev Cell. 2013;25:229–40.
Acknowledgment
The authors thank Ms. Y. Kubota for her wonderful work in preparing the IHC samples, and Ms. T. Shishino and Ms. N. Makikusa for their technical assistance.
Disclosure
Yuichi Hisamatsu, Eiji Oki, Hajime Otsu, Koji Ando, Hiroshi Saeki, Eriko Tokunaga, Shinichi Aishima, Masaru Morita, Yoshinao Oda, and Yoshihiko Maehara had no financial or personal relationships with other people or organizations that could inappropriately influence this work.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
10434_2016_5097_MOESM2_ESM.tif
Supplementary material 2 Figure 1. Immunohistochemical analysis of EGFR and p-AKT in normal gastric tissue. Representative images showing EGFR expression in gastric normal specimens (A, B). Cells were considered EGFR (A) or EGFR+ (B) according to positive immunostaining of the cell membrane. Original magnification: ×200. (TIFF 891 kb).
10434_2016_5097_MOESM3_ESM.tif
Supplementary material 3 Supplementary Figure 2. The level of EGFR expression in NCI-N87 cells. Western blotting analysis using anti-EGFR (sc-03) rabbit polyclonal antibody indicated a band at the predicted size (170 kDa) (TIFF 26 kb).
Rights and permissions
About this article
Cite this article
Hisamatsu, Y., Oki, E., Otsu, H. et al. Effect of EGFR and p-AKT Overexpression on Chromosomal Instability in Gastric Cancer. Ann Surg Oncol 23, 1986–1992 (2016). https://doi.org/10.1245/s10434-016-5097-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5097-3