Abstract
Background
The KRAS mutation status is reportedly correlated with poor survival outcome in patients with colorectal liver metastases (CLM); however, its true prognostic impact and the reason for the poor prognosis remain unclear.
Methods
Data on 163 patients with a known KRAS mutation status who underwent curative resection for CLM were retrospectively reviewed. The long-term survival and site-specific incidence of recurrence were then compared between patients with a KRAS mutation (mtKRAS) and those without a mutation (wtKRAS).
Results
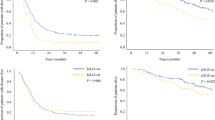
The mtKRAS group had a poorer 3-year disease-specific survival (DSS) rate (59.8 vs. 83.6 %, p = 0.016), 3-year recurrence-free survival (RFS) rate (0 vs. 20.2 %, p = 0.069), and median time to surgical failure (TSF) [18.8 vs. 39.7 months, p = 0.001] than the wtKRAS group. The cumulative incidences of liver recurrence and lung recurrence at 3 years were also higher in the mtKRAS group (76.2 vs. 54.7 %, p = 0.060; and 71.9 vs. 37.3 %, p < 0.001, respectively). A multivariate analysis confirmed that an mtKRAS status had a significant effect on the DSS rate (hazard ratio [HR] 2.9, p = 0.006), RFS (HR 2.0, p = 0.004), TSF (HR 2.4, p < 0.001), liver recurrence (HR 1.7, p < 0.001), and lung recurrence (HR 2.6, p < 0.001). Lung-related unresectable recurrences were more frequent (41 vs. 18 %, p = 0.048) and were associated with an earlier TSF (9.6 vs. 14.0 months, p = 0.14) in the mtKRAS group, regardless of the location of the primary lesions.
Conclusions
mtKRAS is associated with poor survival outcome after CLM resection because of a relatively high incidence of lung recurrence and a relatively short TSF.



Similar content being viewed by others
References
Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27(22):3677–83.
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–18; discussion 18–21.
Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77(7):1254–62.
Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247(1):125–35.
George B, Kopetz S. Predictive and prognostic markers in colorectal cancer. Curr Oncol Rep. 2011;13(3):206–15.
Karagkounis G, Torbenson MS, Daniel HD, et al. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer. 2013;119(23):4137–44.
Stremitzer S, Stift J, Gruenberger B, et al. KRAS status and outcome of liver resection after neoadjuvant chemotherapy including bevacizumab. Br J Surg. 2012;99(11):1575–82.
Vauthey JN, Zimmitti G, Kopetz SE, et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 2013;258(4):619–26; discussion 26–7.
Brudvik KW, Kopetz SE, Li L, Conrad C, Aloia TA, Vauthey JN. Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br J Surg. 2015;102(10):1175–83.
Oba M, Hasegawa K, Matsuyama Y, et al. Discrepancy between recurrence-free survival and overall survival in patients with resectable colorectal liver metastases: a potential surrogate endpoint for time to surgical failure. Ann Surg Oncol. 2014;21(6):1817–24.
Tie J, Lipton L, Desai J, et al. KRAS mutation is associated with lung metastasis in patients with curatively resected colorectal cancer. Clin Cancer Res. 2011;17(5):1122–30.
Gonsalves W, Mahoney M, Sargent D, et al. Patient and tumor chracteristics and BRAF and KRAS mutations in colon cancer, NCCTG/Alliance N0147. J Natl Cancer Inst. 2014;106(7):dju106.
Pollock CB, Shirasawa S, Sasazuki T, Kolch W, Dhillon AS. Oncogenic K-RAS is required to maintain changes in cytoskeletal organization, adhesion, and motility in colon cancer cells. Cancer Res. 2005;65(4):1244–50.
Schramm K, Krause K, Bittroff-Leben A, Goldin-Lang P, Thiel E, Kreuser ED. Activated K-ras is involved in regulation of integrin expression in human colon carcinoma cells. Int J Cancer. 2000;87(2):155–64.
Serova M, Astorgues-Xerri L, Bieche I, et al. Epithelial-to-mesenchymal transition and oncogenic Ras expression in resistance to the protein kinase Cbeta inhibitor enzastaurin in colon cancer cells. Mol Cancer Ther. 2010;9(5):1308–17.
Andreyev HJ, Norman AR, Cunningham D, et al. Kirsten ras mutations in patients with colorectal cancer: the ‘RASCAL II’ study. Br J Cancer. 2001;85(5):692–6.
Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27(5):672–80.
Maughan TS, Adams RA, Smith CG, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377(9783):2103–14.
Richman SD, Seymour MT, Chambers P, et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009;27(35):5931–7.
Mise Y, Zimmitti G, Shindoh J, et al. RAS mutations predict radiologic and pathologic response in patients treated with chemotherapy before resection of colorectal liver metastases. Ann Surg Oncol. 2015;22(3):834–42.
Acknowledgment
This study was supported by a study grant from Okinaka Memorial Institute for Medical Disease (No. 2015-15) and JSPS KAKENHI Grant No. 26861063.
Conflict of interest
Junichi Shindoh, Yujiro Nishioka, Ryuji Yoshioka, Toshitaka Sugawara, Yoshihiro Sakamoto, Kiyoshi Hasegawa, Masaji Hashimoto, and Norihiro Kokudo have no conflicts of interest to declare that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shindoh, J., Nishioka, Y., Yoshioka, R. et al. KRAS Mutation Status Predicts Site-Specific Recurrence and Survival After Resection of Colorectal Liver Metastases Irrespective of Location of the Primary Lesion. Ann Surg Oncol 23, 1890–1896 (2016). https://doi.org/10.1245/s10434-016-5087-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5087-5