Abstract
Background
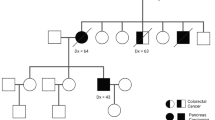
Considering the typical rapid progression and high mortality of pancreatic cancer (PC), early detection may lead to an improved outcome. To date, there is no safe, sensitive, and cost-effective screening strategy to detect PC. Currently, screening is focused on individuals at the highest risk of developing PC based on family history. A high-risk individual is defined as having two or more first-degree relatives with PC, or one first- or second-degree relative with PC with a confirmed mutation in a gene associated with PC. The BRCA2 gene is one of the most common genes linked to pancreatic-only cancer families; however, other hereditary cancer syndromes have also been associated with an increased risk for PC.
Methods
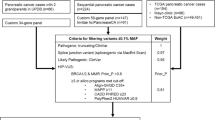
We conducted a retrospective review of pedigrees of families with a pancreatic adenocarcinoma cancer diagnosis held in the statewide Ruth Ann Minner High Risk Family Cancer Registry at the Helen F. Graham Cancer Center and Research Institute, Christiana Care Health System, Newark, DE, USA, from 2002 to 2013. The registry was queried based on how many first-, second-, or third-degree relatives of the proband were affected with PC, genetic testing status, and (if applicable) the results. These data were then categorized into families that meet familial PC (FPC) criteria, defined as two first-degree relatives with PC (FPC families), families that did not meet the FPC definition but had one first-degree relative affected with PC (first-degree families), and probands with PC (probands). Each family was counted only once in the analysis, even if multiple family members were tested.
Results
Our analysis revealed that 175 of 597 families fitting any of the above criteria completed genetic testing. Of this cohort, 52 had pathogenic alterations with nine different genes implicated. Overall, 164 of the 175 families that fitted into any of the three categories previously identified had BRCA1 or BRCA2 testing, either by DNA sequencing or next-generation sequencing via a panel test that included BRCA1/2. BRCA1 pathogenic alterations were noted in 17/164 (10.4 %) and BRCA2 pathogenic alterations were noted in 23/164 (14.0 %). FPC families (n = 46) 42/46 of the FPC families underwent BRCA1/2 testing, and 11/42 (26 % [95 % CI 12.89–39.49]) had pathogenic alterations. Specifically, 4/42 = BRCA1 (9.5 %) and 7/42 = BRCA2 (16.7 %). Additionally, 16/46 of the FPC families underwent exclusively Lynch syndrome (LS) testing, and pathogenic mutations in a mismatch repair protein were identified in 2/16. Specifically, 1/16 = MLH1 (6.3 %) and 1/16 = MSH2 (3.6 %). Overall, a genetic mutation within any gene associated with an increased PC risk was found in 28 % of FPC families. First-degree families (n = 106) 99/106 of the families with one first-degree relative underwent BRCA1/2 testing, and 21/99 (21.2 % [95 % CI 13.16–29.27]) had pathogenic alterations. Specifically, 11/99 = BRCA1 (11.1 %) and 10/99 = BRCA2 (10.1 %). 32/99 first-degree families underwent exclusively LS testing, and pathogenic mutations were identified in 4/32. Specifically, 3/32 = MLH1 (9 %) and 1/32 = MSH6 (3 %). 25/99 of the families pursued panel testing, and pathogenic alterations in any gene were identified in 3/25. Specifically, the mutations were found in 1/25 = ATM (4 %), 1/25 = CHEK2 (4 %), and 1/25 = RAD51D (4 %). Affected probands (n = 23) Lastly, all 23 probands affected with PC pursued genetic testing. Of these, 11/23 were found to have pathogenic alterations. All 23 underwent BRCA1/2 testing, and pathogenic alterations were identified in 8/23 (35 % [95 % CI 15.32–54.25]), specifically 2/23 = BRCA1 (9 %), and 6/23 = BRCA2 (26 %). 10/23 patients underwent panel testing and pathogenic alterations were found in 3/10 (30 %) patients, of whom 1/10 = MSH6 (10 %), 1/10 = ATM (10 %), and 1/10 = TP53 (10 %).
Conclusions
This study demonstrates that a statewide high-risk family cancer registry is an important instrument in studying the risk of PC in families. Our analysis revealed 14 mutations associated with FPC, among which hereditary breast and ovarian cancer and LS were most prevalent. BRCA1 was found to have the same association with PC as BRCA2, which appears unique to our population. We plan to use our knowledge of these mutations in developing a PC screening program.

Similar content being viewed by others
References
Decker GA, Batheja MJ, Collins JM, et al. Risk factors for pancreatic adenocarcinoma and prospects for screening. Gastroenterol Hepatol. 2010;6(4):246–54.
Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–1210.
Jemal A, Siegel R, Ward E, Murray T, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130.
Lynch HT, Voorhees GJ, Lanspa SJ, et al. Pancreatic carcinoma and hereditary nonpolyposis colorectal cancer: a family study. Br J Cancer. 1985;52:271–73.
Risch HA, McLaughlin JR, Cole DE, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kincohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98:1694–706.
Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447–53.
Centers for Disease Control and Prevention. Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion. 2014; http://www.cdc.gov/cancer/npcr/about.htm
Frank TS, Deffenbaugh AM, Reid JE, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol. 2002;20:1480–90.
American Cancer Society. Key statistics about pancreatic cancer. 2014. http://www.cancer.org/cancer/pancreaticcancer/detailedguide/pancreatic-cancer-key-statistics
Cardenes HR, Chiorean EG, Dewitt J, et al. Locally advanced pancreatic cancer: current therapeutic approach. Oncologist. 2006;11(6):612–23.
Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–57.
Jemal A, Murray T, Ward E et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30.
Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995;221:721–31; discussion 731–3.
Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–79.
Brand R, Lynch HT. Hereditary pancreatic adenocarcinoma: a clinical perspective. Med Clin North Am. 2000;84(3):665–75.
Hahn SA, Greenhalf B, Ellis I, et al. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst. 2003;95:214–21.
Nakao A, Harada A, Nonami T, et al. Clinical significance of carcinoma invasion of the extrapancreatic nerve plexus in pancreatic cancer. Pancreas. 1996;12:357–61.
Millikan KW, Deziel DJ, Silverstein JC, et al. Prognostic factors associated with resectable adenocarcinoma of the head of the pancreas. Am Surg. 1999;65:618–23. discussion 623–4.
Ariyama J, Suyama M, Satoh K, et al. Imaging of small pancreatic ductal adenocarcinoma. Pancreas. 1998;16:396–401.
Egawa S, Takeda K, Fukuyama S, et al. Clinicopathological aspects of small pancreatic cancer. Pancreas. 2004;28:235–40.
Canto M. Screening for pancreatic neoplasia in high-risk individuals: who, what, when, how? Clin Gastroenterol Hepatol. 2005;3:S46–S48.
Tersmette AC, Petersen GM, Offerhaus GJ, et al. Increased risk of incident pancreatic cancer among first-degree relatives of patients with familial pancreatic cancer. Clin Cancer Res. 2001;7(3):738–44.
Klein AP. Identifying people at a high risk of developing pancreatic cancer. Nat Rev Cancer. 2013;13:66–74.
McFaul CD, Greenhalf W, Earl J, et al. Anticipation in familial pancreatic cancer. Gut 2006;55:252–8.
Klein AP, Lindström S, Mendelsohn JB, et al. An absolute risk model to identify individuals at elevated risk for pancreatic cancer in the general population. PLoS One. 2013;8(9):e72311.
Bartsch DK, Gress TM, Langer P. Familial pancreatic cancer-current knowledge. Nat Rev Gastroenterol Hepatol. 2012:9:445–53.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Catts, Z.AK., Baig, M.K., Milewski, B. et al. Statewide Retrospective Review of Familial Pancreatic Cancer in Delaware, and Frequency of Genetic Mutations in Pancreatic Cancer Kindreds. Ann Surg Oncol 23, 1729–1735 (2016). https://doi.org/10.1245/s10434-015-5026-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-5026-x