Abstract
Background
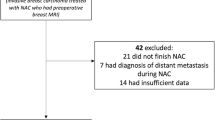
This study evaluated breast imaging procedures for predicting pathologic complete response (pCR = ypT0) after neoadjuvant chemotherapy (NACT) for breast cancer to challenge surgery as a diagnostic procedure after NACT.
Methods
This retrospective, exploratory, monocenter study included 150 invasive breast cancers treated by NACT. The patients received magnetic resonance imaging (MRI), mammography (MGR), and ultrasound (US). The results were classified in three response subgroups according to response evaluation criteria in solid tumors. To incorporate specific features of MRI and MGR, an additional category [clinical near complete response (near-cCR)] was defined. Residual cancer in imaging and pathology was defined as a positive result. Negative predictive values (NPVs), false-negative rates (FNRs), and false-positive rates (FPRs) of all imaging procedures were analyzed for the whole cohort and for triple-negative (TN), HER2-positive (HER2+), and HER2-negative/hormone-receptor-positive (HER2−/HR+) cancers, respectively.
Results
In 46 cases (31 %), pCR (ypT0) was achieved. Clinical complete response (cCR) and near-cCR showed nearly the same NPVs and FNRs. The NPV was highest with 61 % for near-cCR in MRI and lowest with 44 % for near-cCR in MGR for the whole cohort. The FNRs ranged from 4 to 25 % according to different imaging methods. The MRI performance seemed to be superior, especially in TN cancers (NPV 94 %; FNR 5 %). The lowest FPR was 10 % in MRI, and the highest FPR was 44 % in US.
Conclusion
Neither MRI nor MGR or US can diagnose a pCR (ypT0) with sufficient accuracy to replace pathologic diagnosis of the surgical excision specimen.
Similar content being viewed by others
References
Killelea BK, Yang VQ, Mougalian S, Horowitz NR, Pusztai L, Chagpar AB, et al. Neoadjuvant chemotherapy for breast cancer increases the rate of breast conservation: results from the national cancer database. J Am Coll Surg. 2015;220:1063–9. doi:10.1016/j.jamcollsurg.2015.02.011.
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–85.
Von Minckwitz G, Kummel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J, et al. Intensified neoadjuvant chemotherapy in early-responding breast cancer: phase III randomized GeparTrio study. J Natl Cancer Inst. 2008;100:552–62. doi:10.1093/jnci/djn089.
von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–804. doi:10.1200/JCO.2011.38.8595.
Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006;24:2019–27. doi:10.1200/jco.2005.04.1665.
Sardanelli F, Boetes C, Borisch B, Decker T, Federico M, Gilbert FJ, et al. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer. 2010;46:1296–316. doi:10.1016/j.ejca.2010.02.015.
Heys SD, Chaturvedi S. Primary chemotherapy in breast cancer: the beginning of the end or the end of the beginning for the surgical oncologist? World J Surg Oncol. 2003;1:14. doi:10.1186/1477-7819-1-14.
Rea D, Tomlins A, Francis A. Time to stop operating on breast cancer patients with pathological complete response? Eur J Surg Oncol. 2013;39:924–30. doi:10.1016/j.ejso.2013.06.005.
Mamounas EP. Impact of neoadjuvant chemotherapy on locoregional surgical treatment of breast cancer. Ann Surg Oncol. 2015;22:1425–33. doi:10.1245/s10434-015-4406-6.
Tiezzi DG, Andrade JM, Ribeiro-Silva A, Zola FE, Marana HR, Tiezzi MG. HER-2, p53, p21, and hormonal receptors proteins expression as predictive factors of response and prognosis in locally advanced breast cancer treated with neoadjuvant docetaxel plus epirubicin combination. BMC Cancer. 2007;7:36. doi:10.1186/1471-2407-7-36.
Goldstein NS, Decker D, Severson D, Schell S, Vicini F, Margolis J, et al. Molecular classification system identifies invasive breast carcinoma patients who are most likely and those who are least likely to achieve a complete pathologic response after neoadjuvant chemotherapy. Cancer. 2007;110:1687–96. doi:10.1002/cncr.22981.
Gianni L, Zambetti M, Clark K, Baker J, Cronin M, Wu J, et al. Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J Clin Oncol. 2005;23:7265–77. doi:10.1200/JCO.2005.02.0818.
Chagpar AB, Middleton LP, Sahin AA, Dempsey P, Buzdar AU, Mirza AN, et al. Accuracy of physical examination, ultrasonography, and mammography in predicting residual pathologic tumor size in patients treated with neoadjuvant chemotherapy. Ann Surg. 2006;243:257–64. doi:10.1097/01.sla.0000197714.14318.6f.
Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Marinovich ML, Houssami N, Macaskill P, Sardanelli F, Irwig L, Mamounas EP, et al. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J Natl Cancer Instit. 2013;105:321–33. doi:10.1093/jnci/djs528.
National Collaborating Centre for Cancer. Early and locally advanced breast cancer: diagnosis and treatment (NICE Clinical Guidelines, no. 80.). National Collaborating Centre for Cancer (UK), Cardiff, 2009.
Kaufmann M, von Minckwitz G, Mamounas EP, Cameron D, Carey LA, Cristofanilli M, et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2011. doi:10.1245/s10434-011-2108-2.
Senn H. St. Gallen consensus 2013: optimizing and personalizing primary curative therapy of breast cancer worldwide. Breast Care Basel Switzerland. 2013;8:101. doi:10.1159/000351222.
Wockel A, Kreienberg R. First revision of the German S3 Guideline “diagnosis, therapy, and follow-up of breast cancer.” Breast Care. 2008;3:82–6. doi:10.1159/000127509.
Lebeau A, Kreipe H, Dietel M, Schlake W, Kreienberg R. Breast cancer: current recommendations for pathologists on the basis of the S3 guidelines. Der Pathol. 2013;34:293–302; quiz 303–4. doi:10.1007/s00292-013-1763-4.
Heil J, Buehler A, Golatta M, Rom J, Schipp A, Harcos A et al. Do patients with invasive lobular breast cancer benefit in terms of adequate change in surgical therapy from a supplementary preoperative breast MRI? Ann Oncol. 2012;23:98–104. doi:10.1093/annonc/mdr064.
Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Clin Chem. 2003;49:7–18.
Dialani V, Chadashvili T, Slanetz PJ. Role of imaging in neoadjuvant therapy for breast cancer. Ann Surg Oncol. 2015;22:1416–24. doi:10.1245/s10434-015-4403-9.
McGuire KP, Toro-Burguete J, Dang H, Young J, Soran A, Zuley M, et al. MRI staging after neoadjuvant chemotherapy for breast cancer: does tumor biology affect accuracy? Ann Surg Oncol. 2011;18:3149–54. doi:10.1245/s10434-011-1912-z.
De Los Santos JF, Cantor A, Amos KD, Forero A, Golshan M, Horton JK et al. Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer. Translational Breast Cancer Research Consortium trial 017. Cancer. 2013;119:1776–83. doi:10.1002/cncr.27995.
Ciatto S, Houssami N, Apruzzese A, Bassetti E, Brancato B, Carozzi F, et al. Reader variability in reporting breast imaging according to BI-RADS assessment categories (the Florence experience. Breast Edinburgh Scotland. 2006;15:44–51. doi:10.1016/j.breast.2005.04.019.
Disclosures
There are no conflicts of interests (e.g. employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications / registrations, and grants or other funding) by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
B. Schaefgen and M. Mati have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Schaefgen, B., Mati, M., Sinn, H.P. et al. Can Routine Imaging After Neoadjuvant Chemotherapy in Breast Cancer Predict Pathologic Complete Response?. Ann Surg Oncol 23, 789–795 (2016). https://doi.org/10.1245/s10434-015-4918-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4918-0