Abstract
Background
A recent study reported that long non-coding RNA activated by TGF-β (lncRNA-ATB) induced epithelial–mesenchymal transition (EMT) through the transforming growth factor-β (TGF-β)/miR-200s/ZEB axis in hepatocellular carcinoma. Herein, we focused on the clinical significance of lncRNA-ATB in gastric cancer (GC) patients.
Materials and Methods
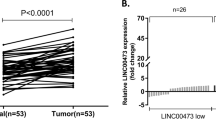
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was performed to examine expression of lncRNA-ATB, miR-200b, and miR-200c in GC tissues (n = 183). Patients were divided into high and low lncRNA-ATB expression groups using a cutoff of lncRNA-ATB/GAPDH ≥0.60 or <0.60 to determine the clinicopathological significance of lncRNA-ATB in GC. Moreover, we evaluated the expression of TGF-β, lncRNA-ATB, miR-200s, and ZEB1 in GC cell lines by qRT-PCR. GC cell lines were treated by recombinant TGF-β1 or TGF-β receptor inhibitor to examine morphologic changes and genetic alterations, such as lncRNA-ATB, miR-200s, and ZEB1 levels, with respect to the EMT phenotype.
Results
The high lncRNA-ATB group experienced a lower overall survival rate compared with the low lncRNA-ATB group, and multivariate analysis indicated that lncRNA-ATB was an independent prognostic factor (hazard ratio 3.50; 95 % CI 1.73–7.44; p = 0.0004). miR-200c levels were lower and ZEB1 levels were higher in the high lncRNA-ATB group than in the low lncRNA-ATB group. Treatment with TGF-β in GC cell lines resulted in morphological EMT changes, upregulation of lncRNA-ATB and ZEB1, and downregulation of miR-200c and CDH1. SB431542 reduced lncRNA-ATB expression.
Conclusion
LncRNA-ATB plays an important role in EMT to promote invasion and metastasis through the TGF-β/miR-200s/ZEB axis, resulting in a poor prognosis in GC. LncRNA-ATB is a novel biomarker of lncRNA, indicative of a poor prognosis in GC patients.



Similar content being viewed by others
References
Nagini S. Carcinoma of the stomach: a review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol. 2012;4:156–69.
Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54.
Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–6.
Gregory PA, Bert AG, Paterson EL, et al. The MiR-200 family and MiR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601.
Wellner U, Schubert J, Burk UC, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–95.
Yu J, Ohuchida K, Mizumoto K, et al. MicroRNA, Hsa-miR-200c, is an independent prognostic factor in pancreatic cancer and its upregulation inhibits pancreatic cancer invasion but increases cell proliferation. Mol Cancer. 2010;9:169.
Hur K, Toiyama Y, Takahashi M, et al. MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut. 2013;62:1315–26.
Liu XG, Zhu WY, Huang YY, et al. High expression of serum MiR-21 and tumor MiR-200c associated with poor prognosis in patients with lung cancer. Med Oncol. 2012;29:618–26.
Cheng H, Zhang L, Cogdell DE, et al. Circulating plasma MiR-141 is a novel biomarker for metastatic colon cancer and predicts poor prognosis. PLoS One. 2011;6:e17745.
Kurashige J, Kamohara H, Watanabe M, et al. MicroRNA-200b regulates cell proliferation, invasion, and migration by directly targeting ZEB2 in gastric carcinoma. Ann Surg Oncol. 2012;19 Suppl 3:S656–64.
Burk U, Schubert J, Wellner U, et al. A reciprocal repression between ZEB1 and members of the MiR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–9.
Prensner JR, Chinnaiyan AM. The emergence of LncRNAs in cancer biology. Cancer Discov. 2011;1:391–407.
Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–46.
Tsai MC, Manor O, Wan Y, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–93.
Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–6.
Matouk IJ, DeGroot N, Mezan S, et al. The H19 non-coding rna is essential for human tumor growth. PLoS One. 2007;2:e845.
Liu XH, Sun M, Nie FQ, et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging MiR-331-3p in gastric cancer. Mol Cancer. 2014;13:92.
Shao Y, Chen H, Jiang X, et al. Low expression of LncRNA-HmlincRNA717 in human gastric cancer and its clinical significances. Tumour Biol. 2014;35(10):9591–5.
Wang Y, Zhang D, Wu K, Zhao Q, Nie Y, Fan D. Long non-coding RNA MRUL promotes ABCB1 expression in multidrug-resistant gastric cancer cell sublines. Mol Cell Biol. 2014;34:3182–93.
Yuan JH, Yang F, Wang F, et al. A long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666–81.
Kurokawa Y, Matsuura N, Kawabata R, et al. Prognostic impact of major receptor tyrosine kinase expression in gastric cancer. Ann Surg Oncol. 2014;21 Suppl 4:S584–90.
Hashiguchi Y, Nishida N, Mimori K, et al. Down-regulation of MiR-125a-3p in human gastric cancer and its clinicopathological significance. Int J Oncol. 2012;40:1477–82.
Tan Z, Jiang H, Wu Y, et al. MiR-185 is an independent prognosis factor and suppresses tumor metastasis in gastric cancer. Mol Cell Biochem. 2014;386:223–31.
Gregory PA, Bracken CP, Smith E, et al. An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mol Biol Cell. 2011;22:1686–98.
Truong HH, Xiong J, Ghotra VP, et al. Beta1 integrin inhibition elicits a prometastatic switch through the TGFbeta-miR-200-ZEB network in E-cadherin-positive triple-negative breast cancer. Sci Signal. 2014;7:ra15.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96.
Acknowledgment
The authors would like to thank Dr. Kohei Miyazono, Department of Molecular Pathology, Graduate School of Medicine, the University of Tokyo, for his technical advice; and Ms. Kazumi Oda, Michiko Kasagi, and Satoko Kono, Kyshu University Beppu Hospital, for their technical support.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2015_4554_MOESM1_ESM.tif
Supplementary material 1 The subgroup analysis of the prognostic significance of the lncRNA-ATB among clinical stages. 22 cases with higher expression of lncRNA-ATB was significantly worse prognosis than 18 cases of lower expression of lncRNA-ATB (p=0.018).(TIFF 80 kb)
Rights and permissions
About this article
Cite this article
Saito, T., Kurashige, J., Nambara, S. et al. A Long Non-coding RNA Activated by Transforming Growth Factor-β is an Independent Prognostic Marker of Gastric Cancer. Ann Surg Oncol 22 (Suppl 3), 915–922 (2015). https://doi.org/10.1245/s10434-015-4554-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4554-8