Abstract
Background and Aims
Compliance with S-1 adjuvant chemotherapy is not satisfactory, and the aim of the present study was to clarify risk factors for the continuation of S-1 after gastrectomy.
Methods
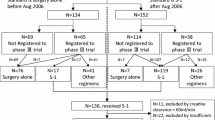
This retrospective study selected patients who underwent curative D2 surgery for gastric cancer, were diagnosed with stage II/III disease, had a creatinine clearance >60 ml/min, and received adjuvant S-1 at our institution between June 2010 and March 2014. The time to S-1 treatment failure (TTF) was calculated.
Results
Fifty-eight patients were selected for the present study. When the TTF curves stratified by each clinical factor were compared using the log-rank test, lean body-mass loss (LBL) of 5 % was regarded as a critical cutoff point. Univariate Cox’s proportional hazard analyses demonstrated that LBL was a significant independent risk factor for continuation. The 6-month continuation rate was 91.7 % in patients with an LBL < 5 %, and 66.3 % for patients with an LBL > 5 % (p = 0.031).
Conclusions
The present study demonstrated that LBL might be an important risk factor for a decrease in compliance to adjuvant chemotherapy with S-1 in patients with stage II/III gastric cancer who underwent D2 gastrectomy. A multicenter, double-blinded, prospective cohort study is necessary to confirm whether LBL would affect adjuvant S-1 continuation.



Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20.
Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomized controlled trial. Lancet. 2012;379:315–21.
Bonadonna G, Valagussa P. Dose-response effect of adjuvant chemotherapy in breast cancer. N Engl J Med. 1981;304:101–5.
Aoyama T, Yoshikawa T, Shirai J, Hayashi T, Yamada T, Tsuchida K, et al. Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2013;20:2000-6.
Fein M, Fuchs KH, Thalheimer A, Freys SM, Heimbucher J, Thiede A. Long-term benefits of Roux-en-Y pouch reconstruction after total gastrectomy: a randomized trial. Ann Surg. 2008;247:759–65.
Lassen K, Soop M, Nygren J, Cox PB, Hendry PO, Spies C, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144:961–969.
Yamada T, Hayashi T, Aoyama T, Shirai J, Fujikawa H, Cho H, et al. Feasibility of enhanced recovery after surgery in gastric surgery: a retrospective study. BMC Surg. 2014;14:41.
Morgan DJ, Bray KM. Lean body mass as a predictor of drug dosage. Implications for drug therapy. Clin Pharmacokinet. 1994;26:292-307.
Roubenoff R, Kehayias JJ. The meaning and measurement of lean body mass. Nutr Rev. 1991;49:163-75.
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115-22.
Lorenzo AD, Andreoli A. Segmental bioelectrical impedance analysis. Curr Opin Clin Nutr Metab Care. 2003;6:551-5.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12.
Aoyama T, Yoshikawa T, Hayashi T, Kuwabara H, Mikayama Y, Ogata T, et al. Risk factors for 6-month continuation of S-1 adjuvant chemotherapy for gastric cancer. Gastric Cancer. 2012;16:133-9.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14: 113-23.
Yamada T, Hayashi T, Cho H, Yoshikawa T, Taniguchi H, Fukushima R, et al. Usefulness of enhanced recovery after surgery protocol as compared with conventional perioperative care in gastric surgery. Gastric Cancer. 2012;15:34–41.
Yoshikawa T, Rino Y, Yukawa N, Oshima T, Tsuburaya A, Masuda M. Neoadjuvant chemotherapy for gastric cancer in Japan: a standing position by comparing with adjuvant chemotherapy. Surg Today. 2014;44:11-21.
Kiyama T, Mizutani T, Okuda T, Fujita I, Tokunaga A, Tajiri T, et al. Postoperative changes in body composition after gastrectomy. J Gastrointest Surg. 2005;9:313-9.
Prado CM, Baracos VE, McCargar LJ, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res. 2007;13:3264-8.
Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patientswith solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629-35.
Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15:2920-6.
Aslani A, Smith RC, Allen BJ, Pavlakis N, Levi JA. The predictive value of body protein for chemotherapy induced toxicity. Cancer. 2000;88:796-803.
Acknowledgments
This work was supported in part by a non-governmental organization, the Kanagawa Standard Anti-Cancer Therapy Support System. The authors express their sincere gratitude to Mrs. Rika Takahashi for her excellent data management.
Disclosure
Toru Aoyama, Taiichi Kawabe, Hirohito Fujikawa, Tsutomu Hayashi, Takanobu Yamada, Kazuhito Tsuchida, Norio Yukawa, Takashi Oshima, Yasushi Rino, Munetaka Masuda, Takashi Ogata, Haruhiko Cho, and Takaki Yoshikawa declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Toru Aoyama and Takaki Yoshikawa have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Aoyama, T., Kawabe, T., Fujikawa, H. et al. Loss of Lean Body Mass as an Independent Risk Factor for Continuation of S-1 Adjuvant Chemotherapy for Gastric Cancer. Ann Surg Oncol 22, 2560–2566 (2015). https://doi.org/10.1245/s10434-014-4296-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-4296-z