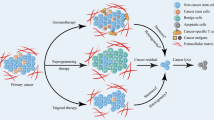
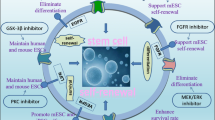
Abstract
Background
We previously generated induced pluripotent stem cells by reprograming adipose stem cells through the introduction of microRNAs targeting four transcription factors (Oct3/4, Sox2, c-Myc, and Klf4). In this study, we aimed to reprogram cancer cells using microRNAs to explore their therapeutic potential.
Methods
Mature microRNAs (mir-302a-d, 369-3p and 5p, and mir-200c, as needed) were introduced into colon cancer cells (DLD-1, RKO, and HCT116) using lipofection.
Results
The transfected cells exhibited an embryonic stem cell-like morphology and expressed the undifferentiated marker genes Nanog, Oct3/4, SOX2, and Klf4, as well as tumor-related antigen-1-60. These cells expressed neurogenic or adipogenic markers, indicating that reprogramming of the cancer cells was partially successful. Moreover, we found that miRNA-expressing DLD-1 cells showed low proliferative activity in vitro and in vivo, accompanied by increased expression of the tumor suppressor genes p16 ink4a and p21 waf1. miRNA-expressing DLD-1 cells also exhibited enhanced sensitivity to 5-fluorouracil, possibly through the downregulation of multidrug-resistant protein 8. The reprogrammed cells from DLD-1, RKO, and HCT116 cells exhibited reduced c-Myc expression, in contrast to the high c-Myc expression in the induced pluripotent cancer cells that were generated using four transcription factors.
Conclusions
Our cancer reprogramming method employing simple lipofection of mature microRNAs is safe and well suited for clinical application, because it avoids integration of exogenous genes into the host genome and allows escape from augmentation of c-Myc gene expression.





Similar content being viewed by others
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–17.
George S, Wang Q, Heinrich MC, et al. Efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: a multicenter phase II trial. J Clin Oncol. 2012;30(19):2401–7.
Vacchelli E, Eggermont A, Galon J, et al. Trial watch: Monoclonal antibodies in cancer therapy. Oncoimmunology. 2013;2(1):e22789.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72.
Miyoshi N, Ishii H, Nagai K, et al. Defined factors induce reprogramming of gastrointestinal cancer cells. Proc Natl Acad Sci USA. 2010;107(1):40–5.
Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–54.
Miyoshi N, Ishii H, Nagano H, et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8(6):633–8.
Takeyama H, Yamamoto H, Yamashita S, et al. Decreased miR-340 expression in bone marrow is associated with liver metastasis of colorectal cancer. Molec Cancer Ther. 2014;13(4):976–85.
Christoffersen NR, Shalgi R, Frankel LB, et al. p53-independent upregulation of miR-34a during oncogene-induced senescence represses MYC. Cell Death Diff. 2010;17(2):236–45.
Yamamura S, Saini S, Majid S, et al. MicroRNA-34a suppresses malignant transformation by targeting c-Myc transcriptional complexes in human renal cell carcinoma. Carcinogenesis. 2012;33(2):294–300.
Shao Y, Qu Y, Dang S, Yao B, Ji M. MiR-145 inhibits oral squamous cell carcinoma (OSCC) cell growth by targeting c-Myc and Cdk6. Cancer Cell Int. 2013;13(1):51.
Wang F, Xia J, Wang N, Zong H. miR-145 inhibits proliferation and invasion of esophageal squamous cell carcinoma in part by targeting c-Myc. Onkologie. 2013;36(12):754–8.
Bork S, Horn P, Castoldi M, Hellwig I, Ho AD, Wagner W. Adipogenic differentiation of human mesenchymal stromal cells is down-regulated by microRNA-369-5p and up-regulated by microRNA-371. J Cell Physiol. 2011;226(9):2226–34.
Lin SL, Chang DC, Ying SY, Leu D, Wu DT. MicroRNA miR-302 inhibits the tumorigenecity of human pluripotent stem cells by coordinate suppression of the CDK2 and CDK4/6 cell cycle pathways. Cancer Res. 2010;70(22):9473–82.
Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22(47):7537–52.
Ohm JE, McGarvey KM, Yu X, et al. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat Genet. 2007;39(2):237–42.
Li H, Collado M, Villasante A, et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460(7259):1136–9.
Nesbit CE, Tersak JM, Prochownik EV. MYC oncogenes and human neoplastic disease. Oncogene. 1999;18(19):3004–16.
Beroukhim R, Mermel CH, Porter D, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463(7283):899–905.
Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–7.
Hacein-Bey-Abina S, Hauer J, Lim A, et al. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2010;363(4):355–64.
Hossain S, Yamamoto H, Chowdhury EH, et al. Fabrication and intracellular delivery of doxorubicin/carbonate apatite nanocomposites: effect on growth retardation of established colon tumor. PloS One. 2013;8(4):e60428.
Acknowledgement
This study was supported in part by a grant-in-aid from the Ministry of Health, Labour, and Welfare to M.M.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2014_4217_MOESM1_ESM.pptx
Supplementary Fig. 1 Expression of endogenous and exogenous miRNAs in colon cancer cell lines. (A): qRT-PCR analysis of endogenous miRNAs in DLD-1, HCT116, Caco2, and RKO cells. miRNA expression was normalized against RNU48 expression, and the mean expression of each miRNA in human embryonic cells was set to 1. mir-200c expression was higher in the DLD-1 and HCT116 cell lines than in human embryonic stem (hES) cells. Expressions of mir-302s and mir-369s were almost undetectable in four colon cancer cell lines. (B): qRT-PCR analysis of miRNAs in DLD-1, pre-transfection and on days 3 and 5 after transfection. (C): qRT-PCR analysis of miRNAs in RKO, pre-transfection and on days 3 and 5 after transfection (PPTX 567 kb)
10434_2014_4217_MOESM2_ESM.pptx
Supplementary Fig. 2 Expression of endogenous and exogenous miRNAs in colon cancer cell lines. (A): On day 30, mRNAs of the colony cells from the RKO line were analyzed using qRT-PCR. Compared to the negative control and parental cells, the miRNA-induced colony cells showed higher levels of Nanog, Oct3/4, Sox2, and KLF4 (p < 0.05 for each). (B): miRNAs in the colony cells induced from the RKO line were analyzed using qRT-PCR. The expression of mir-369s remained high, and those of mir-200c, mir-302a, and mir-302c were increased to some extent, compared with the negative control cells (PPTX 223 kb)
10434_2014_4217_MOESM3_ESM.pptx
Supplementary Fig. 3 Lineage-directed differentiation of miRNA-induced cells. (A): The colonies from the DLD-1 line were grown in standard medium after EB formation, and the cells were isolated on day 15. (B): qRT-PCR analyses showed that the mir-302s level in miRNA-induced cells was reduced to control levels, while the expression of mir-369s remained high. miRNA expression was normalized against RNU48 expression, and the mean expression of each miRNA from human embryonic cells was set as 1. (C): qRT-PCR analysis of mRNAs in miRNA-induced DLD-1 cells. The levels of the differentiation marker mRNAs, including FABP, PPARγ, PAX6, MAP2, albumin, and vimentin, increased. The mRNA expression levels were normalized against GAPDH mRNA expression. *p < 0.05 (PPTX 60 kb)
10434_2014_4217_MOESM4_ESM.pptx
Supplementary Fig. 4 Adipogenic or neurogenic differentiation. (A): ES-like colonies were cultured in a floating state for five days in EB-forming medium composed of DMEM/F12 with 20% knockout-certified serum replacement (KSR; Invitrogen), 2 mM L-glutamine, 100 μM nonessential amino acids, and 100 μM 2-mercaptoethanol (Invitrogen). After five days of EB formation, the miRNA-induced colony-derived cells from the DLD-1 line were cultured in NH AdipoDiff or NDiff N2B27 medium for 16 days, and the cells were examined for differentiation to the adipocyte or neurocyte lineage, respectively. Negative control cells transfected with negative control miRNA were produced under the same culture conditions and treatment procedures. (B): Cells positively stained with Oil Red O. Scale bar: 200 μm. (C): qRT-PCR results indicated approximately 8.3-fold and 6.9-fold increases in the adipogenic marker genes FABP4 and PPARγ in the miRNA-induced cells compared with NC (p < 0.05 for each). The mRNA expression levels were normalized to GAPDH mRNA expression (PPTX 636 kb)
10434_2014_4217_MOESM5_ESM.pptx
Supplementary Fig. 5 (A): Investigations of the neurocyte lineage showed that the cells tended to exhibit an elongated axis foot. They also positively stained for the neurogenic markers (II) GFAP and (IV) TUBB3. (I, III) Phase contrast images. Scale bar: 200 μm. (B): qRT-PCR results indicated 3.3-fold and at 130.4-fold increases in the expression of MAP2 and PAX6, respectively, compared with the negative control. The mRNA expression levels were normalized to GAPDH mRNA expression. p < 0.05 for each (PPTX 125 kb)
10434_2014_4217_MOESM6_ESM.pptx
Supplementary Fig. 6 Proliferation activity and chemosensitivity to 5-FU in miPC cells from RKO. (A): In vitro cell proliferation assays showed significant differences in growth at 72 h and 96 h in miPC cells from RKO, compared to in NC and parental cells (p < 0.01). (B): MTT assays showed that miPC cells from RKO acquired enhanced sensitivity to 5-FU at the indicated concentrations as compared to NC. IC50 values were 0.157 μg/ml in miPC, 0.676 μg/ml in NC, and 0.295 μg/ml in parental cells. p < 0.05 for each (PPTX 288 kb)
10434_2014_4217_MOESM7_ESM.pptx
Supplementary Fig. 7 The mRNA expression levels were normalized to GAPDH mRNA expression. (A): In vivo tumor growth. The miPC cells and DLD-1 parental cells were subcutaneously transplanted into NOD/SCID mice (n = 5). The tumor growth of miPC cells was significantly suppressed compared with that of parental cells on day 21 (p = 0.0339) and 28 (p = 0.018). The negative control tumors grew rapidly, and the mice (n=3) died on day 21 (p<0.05). (B): H&E-stained sections of parental and miPC tumors. No histological difference was noted. Scale Bars: 100 μm (PPTX 139 kb)
10434_2014_4217_MOESM8_ESM.pptx
Supplementary Fig. 8 During adipocyte differentiation, mir-369s levels returned to normal. After five days of EB formation, the miRNA-induced colony-derived cells from DLD-1 were cultured in NH AdipoDiff medium for 16 days. qRT-PCR analyses showed that mir-369s levels, which remained high when cells were cultured with DMEM, decreased during adipocyte differentiation. miRNA expressions were normalized against RNU48 expression. The mean expression of each miRNA in human embryonic cells was set as 1 (PPTX 213 kb)
Rights and permissions
About this article
Cite this article
Miyazaki, S., Yamamoto, H., Miyoshi, N. et al. A Cancer Reprogramming Method Using MicroRNAs as a Novel Therapeutic Approach against Colon Cancer. Ann Surg Oncol 22 (Suppl 3), 1394–1401 (2015). https://doi.org/10.1245/s10434-014-4217-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-4217-1