Abstract
Background
Thrombocytosis is considered an adverse prognostic factor in various malignancies. However, the clinical significance of thrombocytosis in rectal cancer patients is unknown. We investigated the predictive value of thrombocytosis for pathologic tumor response to preoperative chemoradiotherapy (CRT) and oncologic outcomes in patients with rectal cancer.
Methods
A total of 314 patients who underwent preoperative CRT and subsequent rectal resection for rectal cancer were retrospectively evaluated at two tertiary institutions. Univariate and multivariate analyses of the clinical parameters were performed to identify markers predictive of a pathologic complete response (pCR). The Kaplan–Meier method was used to estimate 3-year disease-free and overall survival rates.
Results
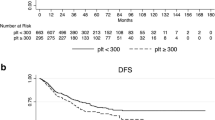
Sixty-nine patients (22 %) had thrombocytosis before CRT, which significantly correlated with a large tumor size and advanced tumor depth. Thirty-nine patients (12.4 %) achieved a pCR. In the multivariate analyses, a platelet count of <370,000/μl (odds ratio 5.483; 95 % confidence interval, 1.271–23.653; P = 0.023) and a carcinoembryonic antigen (CEA) level of <5 ng/dl (odds ratio, 3.084; 95 % confidence interval, 1.291–7.368; P = 0.011) were identified as independent predictive factors for a pCR. Patients with pretreatment thrombocytosis had lower 3-year disease-free (P = 0.037) and overall survival (P = 0.001) rates than patients with normal pretreatment platelet counts.
Conclusions
Thrombocytosis is a negative predictive factor for a pCR and has an adverse impact on survival in rectal cancer. The predictive value of this easily available clinical factor should not be underestimated, and better therapeutic strategies for these tumors are required.

Similar content being viewed by others
References
Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40.
Kuremsky JG, Tepper JE, McLeod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2009;74:673–88.
Negri FV, Campanini N, Camisa R, et al. Biological predictive factors in rectal cancer treated with preoperative radiotherapy or radiochemotherapy. Br J Cancer. 2008;98:143–7.
Watanabe T, Komuro Y, Kiyomatsu T, et al. Prediction of sensitivity of rectal cancer cells in response to preoperative radiotherapy by DNA microarray analysis of gene expression profiles. Cancer Res. 2006;66:3370–4.
Buergy D, Wenz F, Groden C, Brockmann MA. Tumor-platelet interaction in solid tumors. Int J Cancer. 2012;130:2747–60.
Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59:1295–300.
Verheul HM, Jorna AS, Hoekman K, Broxterman HJ, Gebbink MF, Pinedo HM. Vascular endothelial growth factor-stimulated endothelial cells promote adhesion and activation of platelets. Blood. 2000;96:4216–21.
Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–9.
Placke T, Kopp HG, Salih HR. Modulation of natural killer cell anti-tumor reactivity by platelets. J Innate Immun. 2011;3:374–82.
Ikeda M, Furukawa H, Imamura H, et al. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann Surg Oncol. 2002;9:287–91.
Shimada H, Oohira G, Okazumi S, et al. Thrombocytosis associated with poor prognosis in patients with esophageal carcinoma. J Am Coll Surg. 2004;198:737–41.
Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366:610–8.
Sasaki K, Kawai K, Tsuno NH, Sunami E, Kitayama J. Impact of preoperative thrombocytosis on the survival of patients with primary colorectal cancer. World J Surg. 2012;36:192–200.
Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Preoperative thrombocytosis is associated with survival after surgery for colorectal cancer. J Surg Oncol. 2012;106:887–91.
Tomita M, Shimizu T, Hara M, Ayabe T, Onitsuka T. Prognostic impact of thrombocytosis in resectable non-small cell lung cancer. Interact Cardiovasc Thorac Surg. 2008;7:613–5.
Park JS, Choi GS, Jun SH, Hasegawa S, Sakai Y. Laparoscopic versus open intersphincteric resection and coloanal anastomosis for low rectal cancer: intermediate-term oncologic outcomes. Ann Surg. 2011;254:941–6.
Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23.
Troxler M, Dickinson K, Homer-Vanniasinkam S. Platelet function and antiplatelet therapy. Br J Surg. 2007;94:674–82.
Jurasz P, Alonso-Escolano D, Radomski MW. Platelet–cancer interactions: mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br J Pharmacol. 2004;143:819–26.
Italiano JE Jr., Richardson JL, Patel-Hett S, et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–33.
Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. Biochem J. 2000;349:385–402.
Mori K, Kitayama J, Shida D, Yamashita H, Watanabe T, Nagawa H. Lysophosphatidic acid-induced effects in human colon carcinoma DLD1 cells are partially dependent on transactivation of epidermal growth factor receptor. J Surg Res. 2006;132:56–61.
Shida D, Kitayama J, Yamaguchi H, et al. Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer Res. 2003;63:1706–11.
Das P, Skibber JM, Rodriguez-Bigas MA, et al. Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer. Cancer. 2007;109:1750–5.
Restivo A, Zorcolo L, Cocco IM, et al. Elevated CEA levels and low distance of the tumor from the anal verge are predictors of incomplete response to chemoradiation in patients with rectal cancer. Ann Surg Oncol. 2013;20:864–71.
Huh JW, Kim HR, Kim YJ. Clinical prediction of pathological complete response after preoperative chemoradiotherapy for rectal cancer. Dis Colon Rectum. 2013;56:698–703.
Kawai K, Kitayama J, Tsuno NH, Sunami E, Watanabe T. Thrombocytosis before pre-operative chemoradiotherapy predicts poor response and shorter local recurrence-free survival in rectal cancer. Int J Colorectal Dis. 2013;28:527–35.
Song S, Hong JC, McDonnell SE, et al. Combined modality therapy for rectal cancer: the relative value of posttreatment versus pretreatment CEA as a prognostic marker for disease recurrence. Ann Surg Oncol. 2012;19:2471–6.
Wallin U, Rothenberger D, Lowry A, Luepker R, Mellgren A. CEA—a predictor for pathologic complete response after neoadjuvant therapy for rectal cancer. Dis Colon Rectum. 2013;56:859–68.
Acknowledgment
Hye Jin Kim designed the study and wrote the manuscript; Gyu-Seog Choi and Toshiaki Watanabe, both were responsible for correspondence and the study proposal; Jun Seok Park, SooYeun Park, and Kazushige Kawai provided data collection and analysis, and approved the final manuscript.
Disclosure
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2014_3988_MOESM1_ESM.tif
Supplement 1. Kaplan–Meier cumulative survival curves relative to thrombocytosis groupings categorized with respect to pre- and post-CRT platelet counts; those with pre-CRT and post-CRT platelet counts <370,000/µL (Group A); those with pre-CRT platelet counts ≥370,000/µL and post-CRT platelet counts <370,000/µL (Group B); and those with post-CRT platelet counts ≥370,000/µl regardless of the pre-CRT platelet count (Group C). (A) Disease-free survival (TIFF 61206 kb)
10434_2014_3988_MOESM2_ESM.tif
Supplement 2. Kaplan–Meier cumulative survival curves relative to thrombocytosis groupings categorized with respect to pre- and post-CRT platelet counts; those with pre-CRT and post-CRT platelet counts <370,000/µL (Group A); those with pre-CRT platelet counts ≥370,000/µL and post-CRT platelet counts <370,000/µL (Group B); and those with post-CRT platelet counts ≥370,000/µl regardless of the pre-CRT platelet count (Group C). (B) Overall Survival. (TIFF 61206 kb)
Rights and permissions
About this article
Cite this article
Kim, H.J., Choi, GS., Park, J.S. et al. Clinical Significance of Thrombocytosis Before Preoperative Chemoradiotherapy in Rectal Cancer: Predicting Pathologic Tumor Response and Oncologic Outcome. Ann Surg Oncol 22, 513–519 (2015). https://doi.org/10.1245/s10434-014-3988-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-3988-8