Abstract
Purpose
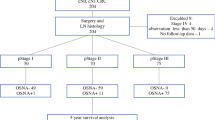
To investigate the prognostic value of occult metastases detected by quantitative measurements of candidate biomarkers in sentinel lymph nodes (SLNs) from patients curatively resected for colon cancer.
Methods
Resection specimens from consecutive patients undergoing surgery for localized colon cancer were subjected to ex vivo SLN mapping. SLNs were examined for the presence of metastases by routine hematoxylin–erythrosin–safranin staining and by cytokeratin 20 (CK20) and mucin 2 (MUC2) mRNA quantification. The patients were stratified according to KRAS and BRAF mutation status and microsatellite instability status in their primary tumors. Survival end points were analyzed by Kaplan–Meier survival estimates and log-rank tests.
Results
A total of 817 SLNs were identified in 206 (97 %) of the 213 included patients. Routine histological examination of SLNs and other regional lymph nodes identified 63 patients with positive nodes (pN+), of which 42 (67 %) were positive in one or more SLNs (sensitivity 67 %, false-negative rate 33 %). On the basis of the CK20 and MUC2 mRNA levels in SLNs, occult metastases were suggested in 86 (60 %) and 52 (36 %) of the 143 otherwise LN-negative (pN0) patients, respectively. Survival analysis with a median 3.6-year follow-up revealed that MUC2 mRNA quantification had significant prognostic value in SLNs from all patients; however, occult SLN metastasis detection did not.
Conclusions
Occult SLN metastases detected by CK20 and MUC2 mRNA quantification had limited prognostic value.



Similar content being viewed by others
References
Edge S, Byrd D, Compton C, et al., editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. Lancet. 1995;345:939–44.
Wolmark N, Fisher B, Wieand HS. The prognostic value of the modifications of the Dukes’ C class of colorectal cancer. An analysis of the NSABP clinical trials. Ann Surg. 1986;203:115–22.
Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–51.
Figueredo A, Charette ML, Maroun J, et al. Adjuvant therapy for stage II colon cancer: a systematic review from the Cancer Care Ontario Program in Evidence-based Care’s Gastrointestinal Cancer Disease Site Group. J Clin Oncol. 2004;22:3395–407.
Andre T, Sargent D, Tabernero J, et al. Current issues in adjuvant treatment of stage II colon cancer. Ann Surg Oncol. 2006;13:887–98.
Greene FL, Stewart AK, Norton HJ. A new TNM staging strategy for node-positive (stage III) colon cancer: an analysis of 50,042 patients. Ann Surg. 2002;236:416–21.
Iddings D, Ahmad A, Elashoff D, et al. The prognostic effect of micrometastases in previously staged lymph node negative (N0) colorectal carcinoma: a meta-analysis. Ann Surg Oncol. 2006;13:1386–92.
Doekhie FS, Kuppen PJ, Peeters KC, et al. Prognostic relevance of occult tumour cells in lymph nodes in colorectal cancer. Eur J Surg Oncol. 2006;32:253–8.
Nicastri DG, Doucette JT, Godfrey TE, et al. Is occult lymph node disease in colorectal cancer patients clinically significant? A review of the relevant literature. J Mol Diag. 2007;9:563–71.
van der Pas MH, Meijer S, Hoekstra OS, et al. Sentinel-lymph-node procedure in colon and rectal cancer: a systematic review and meta-analysis. Lancet Oncol. 2011;12:540–50.
de Haas RJ, Wicherts DA, Hobbelink MG, et al. Sentinel lymph node mapping in colon cancer: current status. Ann Surg Oncol. 2007;67:347–55.
Lim SJ, Feig BW, Wang H, et al. Sentinel lymph node evaluation does not improve staging accuracy in colon cancer. Ann Surg Oncol. 2008;15:46–51.
Koyanagi K, Bilchik AJ, Saha S, et al. Prognostic relevance of occult nodal micrometastases and circulating tumor cells in colorectal cancer in a prospective multicenter trial. Clin Cancer Res. 2008;14:7391–6.
Nordgard O, Oltedal S, Kørner H, et al. Quantitative RT-PCR detection of tumor cells in sentinel lymph nodes isolated from colon cancer patients with an ex vivo approach. Ann Surg. 2009;249:602–7.
Dahl O. Adjuvant behandling ved operabel kolorektalcancer. NGICG, 2007. http://ngicg.no/. Accessed 27 Sep 2011.
Nordgard O, Oltedal S, Korner H, et al. The potential of cytokeratin 20 and mucin 2 mRNA as metastasis markers in regional lymph nodes of colon cancer patients investigated by quantitative RT-PCR. Int J Colorectal Dis. 2009;24:261–268.
Thorstensen L, Lind GE, Løvig T, et al. Genetic and epigenetic changes of components affecting the WNT pathway in colorectal carcinomas stratified by microsatellite instability. Neoplasia. 2005;7:99–108.
Wu Q, Lothe RA, Ahlquist T, et al. DNA methylation profiling of ovarian carcinomas and their in vitro models identifies HOXA9, HOXB5, SCGB3A1, and CRABP1 as novel targets. Mol Cancer. 2007;6:45.
Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–8.
Gilje B, Heikkila R, Oltedal S, et al. High-fidelity DNA polymerase enhances the sensitivity of a peptide nucleic acid clamp PCR assay for K-ras mutations. J Mol Diagn. 2008;10:325–31.
Oltedal S, Gilje B, Kørner H, et al. Detection of occult metastases in sentinel lymph nodes from colon cancer patients by K-ras mutation peptide nucleic acid clamp PCR. Ann Surg. 2010;251:1087–91.
Ahlquist T, Bottillo I, Danielsen SA, et al. RAS signaling in colorectal carcinomas through alteration of RAS, RAF, NF1, and/or RASSF1A. Neoplasia. 2008;10:680–6.
Cahill RA, Leroy J, Marescaux J. Could lymphatic mapping and sentinel node biopsy provide oncological providence for local resectional techniques for colon cancer? A review of the literature. BMC Surg. 2008;8:17.
Nordgard O, Smaaland R. SLN mapping in colorectal cancer. Lancet Oncol. 2011;12:990.
Waldman SA, Hyslop T, Schulz S, et al. Association of GUCY2C expression in lymph nodes with time to recurrence and disease-free survival in pN0 colorectal cancer. JAMA. 2009;301:745–52.
Doekhie FS, Mesker WE, Kuppen PJ, et al. Detailed examination of lymph nodes improves prognostication in colorectal cancer. Int J Cancer. 2010;126:2644–52.
Faerden AE, Sjo OH, Bukholm IRK, et al. Lymph node micrometastases and isolated tumor cells influence survival in stage I and II colon cancer. Dis Colon Rectum. 2011;54:200–6.
Hyslop T, Weinberg DS, Schulz S, et al. Occult tumor burden predicts disease recurrence in lymph node–negative colorectal cancer. Clin Cancer Res. 2011;17:3293–303.
Ohlsson L, Israelsson A, Oberg A, et al. Lymph node CEA and MUC2 mRNA as useful predictors of outcome in colorectal cancer. Int J Cancer. 2012;130:1833–43.
Rosenberg R, Hoos A, Mueller J, et al. Prognostic significance of cytokeratin-20 reverse transcriptase polymerase chain reaction in lymph nodes of node-negative colorectal cancer patients. J Clin Oncol. 2002;20:1049–55.
Wong JH, Johnson DS, Namiki T, et al. Validation of ex vivo lymphatic mapping in hematoxylin–eosin node-negative carcinoma of the colon and rectum. Ann Surg Oncol. 2004;11:772–7.
Faerden AE, Sjo OH, Andersen SN, et al. Sentinel node mapping does not improve staging of lymph node metastasis in colonic cancer. Dis Colon Rectum. 2008;51:891–6.
Wiese D, Sirop S, Yestrepsky B, et al. Ultrastaging of sentinel lymph nodes (SLNs) vs. non-SLNs in colorectal cancer: do we need both? Am J Surg. 2010;199:354–8.
Acknowledgment
This work was supported by the Norwegian Cancer Society, the Western Norway Regional Health Authority, the Folke Hermansen Fund, and Stavanger University Hospital.
Conflict of interest
Reino Hekkilä is presently employed at Roche Norway, AS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nordgård, O., Oltedal, S., Aasprong, O.G. et al. Prognostic Relevance of Occult Metastases Detected by Cytokeratin 20 and Mucin 2 mRNA Levels in Sentinel Lymph Nodes from Colon Cancer Patients. Ann Surg Oncol 19, 3719–3726 (2012). https://doi.org/10.1245/s10434-012-2454-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-012-2454-8